copy the linklink copied!1. Defining the challenge of managing pharmaceuticals in water
This chapter characterises the diversity of pharmaceuticals, and their sources, mixtures and various entry pathways into the environment. It also summarises recent literature on the impacts of pharmaceuticals on water quality, human health and freshwater ecosystems, and makes the case for policy action.
copy the linklink copied!1.1. Key messages
Pharmaceuticals are essential for human and animal health but have been recognised as an environmental concern when their residues enter freshwater systems. Due to demographic and epidemiological changes, the usage of pharmaceuticals has rapidly increased in OECD member countries. About 2 000 active pharmaceutical ingredients are being administered worldwide in prescription medicines, over-the-counter therapeutic drugs and veterinary drugs (Burns et al., 2018[1]).
The vast majority of them have not been evaluated for their occurrence, fate and possible impacts on water quality, human health and freshwater ecosystems. Municipal wastewater effluent is considered the most dominant pathway to freshwater bodies globally, however, emissions from manufacturing plants, hospitals, and intensive agriculture and aquaculture practices can be important sources locally.
As pharmaceuticals are designed to interact with living systems at low doses, even low environmental concentrations can be of concern. There is growing evidence of their occurrence in the environment and potential negative impacts. For example, steroid hormones in oral contraceptives have been proven in the laboratory to cause the feminisation of fish; psychiatric drugs, such as fluoxetine, can alter fish behaviour; and the use and discharge of antibiotics to water bodies is linked to antimicrobial resistance – a global health crisis. Some researchers stress the lack of human risk assessment regarding long-term and low-levels of pharmaceutical mixtures towards sensitive sub-populations (e.g. pregnant women, foetuses and children).
copy the linklink copied!1.2. Introduction
Pharmaceuticals are synthetic or natural chemical compounds that are manufactured for use as prescription medicines, over-the-counter therapies, veterinary drugs and illicit drugs. Pharmaceuticals contain active ingredients that have been designed to have pharmacological effects and confer net benefits to society. The incidence of pharmaceuticals in the environment and the water cycle at trace levels (in the range of nanograms to low micrograms per litre) has been widely discussed and published in literature in the past decade. The increase in detection is largely attributable to advances in analytical techniques and instrumentation (WHO, 2012[2]) and the continuous increased use of pharmaceuticals.
About 2 000 active pharmaceutical ingredients (APIs) are being administered worldwide in prescription medicines, over-the-counter therapeutic drugs and veterinary drugs (Burns et al., 2018[1]). Their active ingredients comprise a variety of molecules produced by pharmaceutical companies in both the industrialised and the developing world at a rate of 100,000 tons per year (Weber et al., 2014[3]). The annual rate of increase in the development and approval of new APIs over the past five years has averaged about 43 in the United States (in 2018, the number approved was 59 – a record year) (Mullard, 2019[4]).
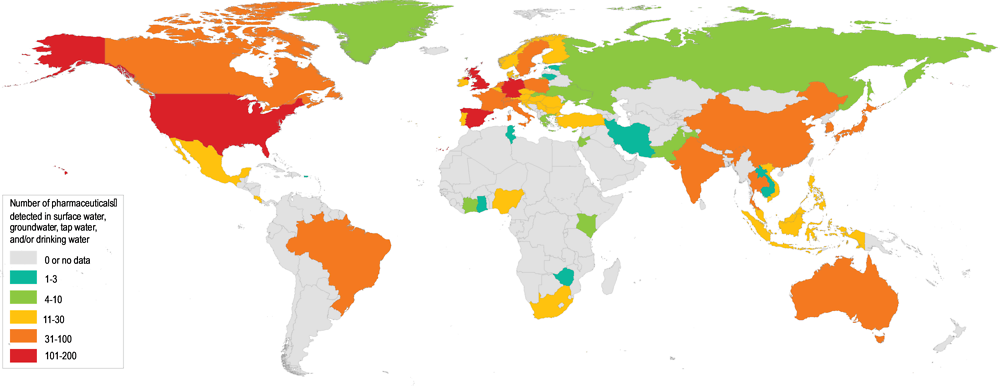
The continuous and increased production and use of pharmaceuticals has led to their widespread occurrence in the aquatic environment across the globe (Figure 1.1). Many APIs have been found worldwide in soils, biota, sediments, surface water, groundwater and drinking water. For example, research by Boxall and Wilkinson (forthcoming) tested 711 river sites in 72 countries for the presence of antibiotics and found antibiotics in 65% of them. In 111 of the sites, the concentrations of antibiotics exceeded safe levels, with the worst cases more than 300 times over the safe limit set by the AMR Industry Alliance.
As a consequence of APIs in the environment, there are recognised adverse effects on aquatic organisms, and recognised and undefined, long-term effects for humans through consumption of contaminated drinking water or food and antimicrobial resistance.
Pharmaceuticals in the environment are a challenge to manage for the following reasons:
Active pharmaceutical ingredients are designed to interact with a living system and produce a pharmacological response at low doses, which makes them of environmental concern even at low concentrations. They are often designed to easily pass biological membranes and can interact with target molecules across a range of organisms. When exposed to non-target organisms in the environment, unintentional harmful impacts may occur. Furthermore, for APIs to be active at the target site, excess doses are regularly required to account for losses due to low uptake, availability issues and metabolisation of the API.
Pharmaceuticals are designed to be stable (persistent or “pseudo‐persistent”) in order to reach and interact with target molecules (Khetan and Collins, 2007[6]). This means that either they are very slow to degrade or their constant use leads to continuous release into the environment at rates exceeding degradation rates (Bernhardt, Rosi and Gessner, 2017[7]). Hence, the chemical design elements for an effective pharmaceutical are contrary to what is desirable in the environment.
Incentives are lacking for replacement of existing pharmaceuticals with “greener” alternatives. But in an era of growing human and ecosystem health threats from antimicrobial resistance and changes in ecosystem functioning, this neglect of environmental impacts of pharmaceuticals may be misplaced.
Conventional wastewater treatment plants (WWTPs) are not designed to remove pharmaceuticals (although some APIs are removed by conventional wastewater treatment to a limited extent). Furthermore, veterinary pharmaceuticals used in agriculture and aquaculture can enter water bodies directly or via surface runoff (diffuse pollution).
For most wildlife, exposure to pharmaceuticals in the environment could be long-term, potentially occurring via multiple exposure routes, and involving a mixture of substances.
Managing the risks of pharmaceuticals in the environment requires a multi-disciplinary and multi-stakeholder approach.
In order to manage the risks of pharmaceuticals in the environment, it is first necessary to understand the origins, entry-pathways, sinks and concentration patterns of pharmaceuticals in the environment, and their effects on human and ecosystem health. The remainder of this chapter aims to do just that.
copy the linklink copied!1.3. Origins, entry-pathways, sinks and concentration patterns of pharmaceuticals in the environment
1.3.1. A typology for pharmaceuticals in the environment
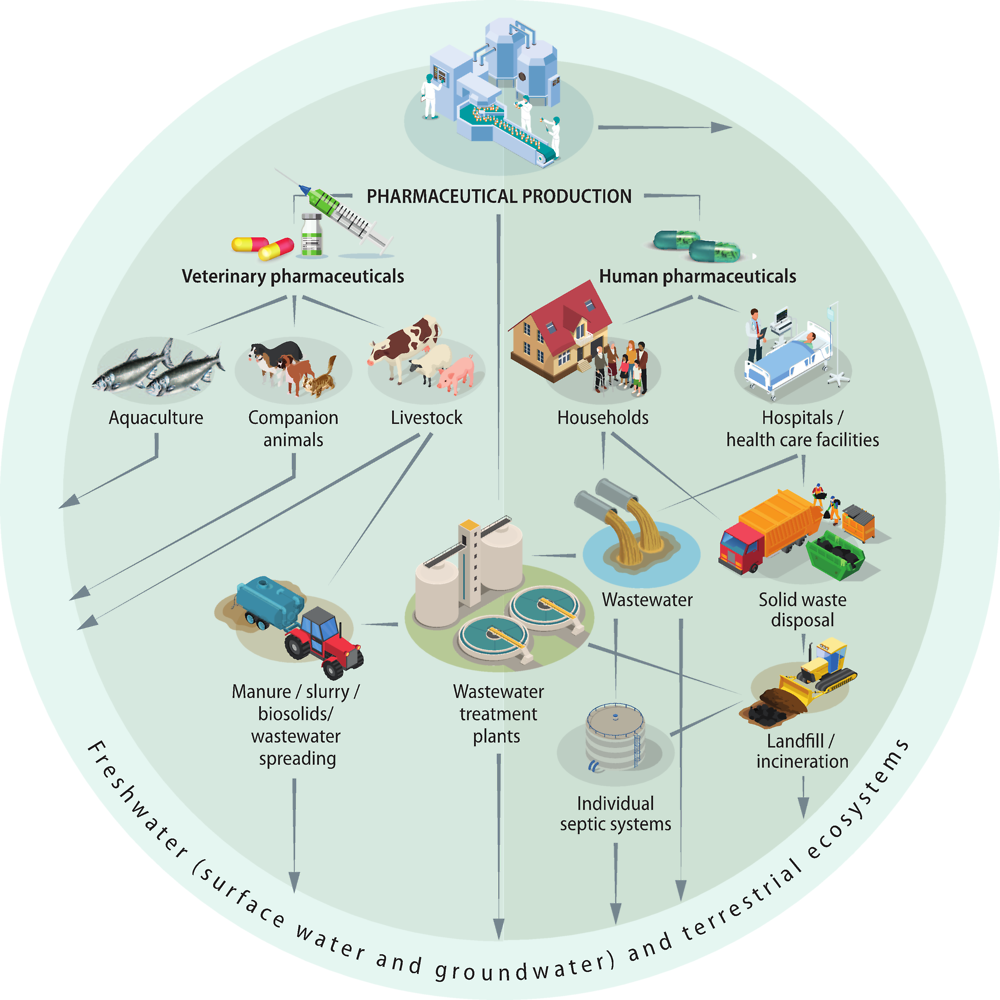
As mentioned in the previous section, human and veterinary pharmaceuticals, and their metabolites and transformation products, are ubiquitously present in water bodies. Pharmaceuticals are present in the environment as a consequence of pharmaceutical production and formulation, patient use, use in food production and improper disposal. After passing through the human or animal body, APIs are excreted either in an unchanged active form or as metabolites, which may be active or inactive, and have the potential for further breakdown into numerous transformation products in WWTPs or in the environment. Pharmaceuticals can disperse through the environment via multiple pathways as illustrated in Figure 1.2. The presence of pharmaceuticals in freshwater and terrestrial ecosystems can result in the uptake of pharmaceuticals into wildlife, and have the potential to bioaccumulate (Arnold et al., 2014[8]). Humans can subsequently be exposed through drinking water, and ingestion of pharmaceutical residues in plant crops, fish, dairy products and meat.
The concentrations and impacts of pharmaceuticals in the environment depend on a combination of variables, including their use, and the toxicity, degradation, persistence and mobility properties of the pharmaceutical; source and timing of pollution; WWTP technology, operation and removal efficiency; agriculture and veterinary practices; sensitivity of the receiving environment and exposure history; and stochastic environmental conditions (Table 1.1).
1.3.2. Sources and entry-pathways
The key sources of pharmaceuticals and their metabolites in the environment are (Kümmerer, 2009[9]; Monteiro and Boxall, 2010[10]; Lapworth et al., 2012[11]; Larsson, 2014[12]):
Pharmaceutical manufacturing industry, including industrial wastewater discharge and solid wastes containing drugs, and stormwater runoff carrying powdered drugs.
Consumers/households, including excretion and inappropriate disposal of pharmaceuticals to wastewater systems (central or individual), which are then discharged (treated or untreated, including as combined sewer overflow) or leaked (from leaky sewers and septic systems) to the environment. Household solid waste containing pharmaceuticals is another source of pharmaceuticals in the environment via landfills.
Hospitals, including the discharge of wastewater and solid wastes.
Agriculture and aquaculture, including: residual hormones and other drugs injected to poultry, cattle and fish; antibiotics added to livestock feed and waters; and runoff of livestock manure and slurry, and recycled wastewater and biosolids.
While the contribution of each emission source varies across regions and type of pharmaceuticals, it is generally accepted that, globally, the main route for human pharmaceuticals to the aquatic environment is via discharge of untreated or treated wastewater from households (Michael et al., 2013[13]; Weber et al., 2014[3]; Heberer and Feldmann, 2005[14]; Verlicchi, Al Aukidy and Zambello, 2012[15]; Verlicchi et al., 2010[16]). For veterinary pharmaceuticals, agriculture is the most significant source of water contamination via land application of livestock manure and slurry as irrigation water and fertiliser (Boxall, 2012[17]). For example, while practices differ across countries, globally more than 70% of all antimicrobials sold in 2013 were used in the agriculture sector, mainly as a growth promoter but also as a substitute for good hygiene (Van Boeckel, 2017[18]).
Pharmaceutical manufacturing
Pharmaceutical manufacturing facilities have been shown to release APIs into nearby streams and can be important pollution hotspots locally (Weber et al., 2014[3]; Larsson, de Pedro and Paxeus, 2007[19]) Extremely high pharmaceutical concentrations, in the order of mg/L have been detected in some industrial effluents and recipient streams, for example in India, China, USA, Korea and Israel (Larsson, 2014[12]). Environmental concentrations of pharmaceuticals discharged from manufacturing plants are generally much higher than excretion from humans (via WWTPs), and in some cases can greatly exceed toxic threshold concentrations. Although pollution from manufacturing is less widespread, discharges that promote the development of drug resistant microorganisms can have global consequences from a human health perspective (i.e. risk associated with antimicrobial resistance) (Larsson, 2014[12]).
Most API production takes place in emerging economies, mainly in Central and South America and in the Asia-Pacific region, which have become the global API production hubs. Consequently, this is where most of the pollution related to manufacturing occurs (BIO Intelligence Service, 2013[20]). Emissions from manufacturing facilities at production hubs leads to substantial discharges (in the order of several µg/L to mg/L) (Larsson, 2014[21]) causing contamination of surface water, sediment (Kristiansson et al., 2011[22]), groundwater and drinking water wells (Fick et al., 2009[23]). For example, the effluent of one WWTP serving 90 manufacturers of bulk drugs in Patancheru, Hyderabad, India was found to have levels of ciprofloxacin as high as 32 mg/L (Larsson, de Pedro and Paxeus, 2007[24]), which is considerably higher than levels found in the blood of patients taking antibiotics. The most striking finding from this Indian WWTP was that all bacteria isolated inside the facility were found to be multi-resistant to antibiotics which can have global consequences for the spread of antimicrobial resistance (Johnning et al., 2013[25]; Marathe et al., 2013[26]) (for introduction to antimicrobial resistance see Box 1.7). Furthermore, in the receiving lake and up to 17 km downstream from the WWTP, the levels of resistant genes were high (Kristiansson et al., 2011[22]).
Within developed nations, emissions of pharmaceuticals from manufacturing facilities (including facilities that formulate finished pharmaceutical products from APIs imported in emerging economies) are less widespread compared to emissions from WWTPs. However, pollution downstream of manufacturers has been observed at EU monitored sites (e.g. the Rhine, Lake Leman) (BIO Intelligence Service, 2013[20]) and when investigating effluents in the U.S (Scott et al., 2018[27]) and other OECD countries (Larsson, 2014[21]).
Household and hospital consumption (usage)
The consumption stage of the life cycle of pharmaceuticals is considered the greatest contributor to the environmental load of pharmaceutical residues in water in OECD countries. Pharmaceuticals administrated to humans or animals are excreted via urine and faeces, with an estimated 30 to 90% of oral doses generally excreted as active substances (BIO Intelligence Service, 2013[20]). However, the nature and amount of medicinal residues mainly depend on the volumes and nature of the administered substances, their modes of administration, and metabolisation rates. The usage of pharmaceuticals varies across regions in terms of commonly used substances, clinical practice and prescription patterns.
The dominant source of human pharmaceuticals in the environment is from households; pharmaceutical use in hospitals and nursing homes is estimated to account for a few percent of the total release from a city (Azuma et al., 2016[28]; Lacorte et al., 2018[29])). However, this varies widely depending on the type of pharmaceutical. Some substances are only intended for use in hospitals, whilst others are taken, or excreted, at home. For instance, hospitals are the dominant (70-90%) source of anti-cancer drugs, endocrine therapy and contrast media (although they may be excreted at home after discharge from hospital), while households are the dominant source of painkillers, blood pressure medicine (BIO Intelligence Service, 2013[20]) and anti-inflammatories (Daughton and Ruhoy, 2009[30]). Knowledge gaps exist regarding the sales and consumption of over-the-counter and self-prescribed pharmaceuticals.
Municipal WWTPs collect and concentrate a variety of human pharmaceuticals (and their metabolites) administered in households, hospitals and elderly care homes. Conventional WWTPs are not designed to remove pharmaceuticals or their metabolites (Box 1.1); WWTPs are primarily designed to remove pathogens, suspended solids and organic and inorganic matter, rather than the removal of the increasing numbers of modern chemicals, including pharmaceuticals at low concentrations (Melvin and Leusch, 2016[31]). WWTPs can therefore release APIs and metabolites to the environment, depending on the level and type of wastewater treatment, (Yang et al., 2017[32]) in unchanged forms or as transformation products. Unused medicines that are improperly disposed of in sinks and toilets also end up in municipal wastewater.
The degree of removal of different pharmaceuticals in wastewater treatment plants (WWTPs) is highly variable depending on the type of pharmaceuticals entering the system, their physico-chemical properties, and the removal efficiency of WWTP technology. There are large discrepancies in removal efficiencies of pharmaceuticals in WWTPs between countries, and even between WWTPs within the same country (Tran, Reinhard and Gin, 2018[33]). No single technique has been found to remove all relevant pollutants from wastewater (Hollender et al., 2009[34]; Melvin and Leusch, 2016[31]; Behera et al., 2011[35]) (Verlicchi, Al Aukidy and Zambello, 2012[15]).
In a review by Deblonde et al. (2011[36]), the removal rates of pharmaceuticals following wastewater treatment ranged from 0% (contrast media) to 97% (psychostimulant). The removal rate for antibiotics was about 50%. Analgesics, anti-inflammatories and beta-blockers were some of the most resistant to treatment (30–40% removal rate).
In the UK, as part of the Chemical Investigation Programme, the concentrations of 19 APIs and 4 metabolites were monitored 20 times in the influent and effluent of 45 WWTPs over a two year period (2015-2017). The results, published by Comber et al. (2018[37]), show that the majority of substances studied were removed to a high degree, although with significant variation, both within and between WWTPs. Poorer removal (between influent and effluent) was observed for ethinyloestradiol, diclofenac, propranolol, the macrolide antibiotics, fluoxetine, tamoxifen and carbamazepine. All except the last two of these substances were present in effluents at concentrations higher than their respective estimated PNEC. Based on available dilution data, as many as 890 WWTPs in the UK (approximately 13% of all WWTPs) may cause exceedances of estimated riverine PNECs after mixing of their effluents with receiving waters. If the estimated PNECs are a guide to regulatory limits, then there is potential for localised non-compliance in surface waters, at least in the case of ethinyloestradiol, diclofenac, ibuprofen, propranolol and the macrolide antibiotics (Comber et al., 2018[37])
Sources: (Comber et al., 2018[37]) (Deblonde, Cossu-Leguille and Hartemann, 2011[36]) (Hollender et al., 2009[34]; Melvin and Leusch, 2016[31]; Behera et al., 2011[35]) (Verlicchi, Al Aukidy and Zambello, 2012[15]) (Gardner et al., 2012[38]).
Pharmaceuticals improperly disposed of in the household garbage end up in landfills which can eventually be transferred to surface or groundwater bodies if there is no collection of landfill leachate (Tong, Peake and Braund, 2011[39]; Saad et al., 2017[40]; Barnes et al., 2004[41]). A large number of the prescription items dispensed every year are not taken or administered, and become waste. For example, in the US, it is estimated that about one-third of the four billion prescription items annually become waste (Product Stewardship Council, 2018[42]). Over-prescription, self-medication (over-the-counter pharmaceuticals) and misdiagnosis of symptoms can increase the amount of APIs administered and improperly disposed of. There is little information about the contribution of improper disposal of pharmaceuticals in relation to other sources of pollution (BIO Intelligence Service, 2013[20]).
Agriculture and aquaculture
Although veterinary pharmaceuticals may benefit the health and welfare of domestic animals and the efficiency of intensive food animal and fish production, they can contaminate the water resources through manufacturing, treatment of animals, and disposal of carcasses, offal, effluent, manure and unused products (Boxall, 2010[43]). Veterinary pharmaceuticals used in aquaculture directly enter surface waters (Weber et al., 2014[3]).
A wide range of veterinary pharmaceuticals are used in the agriculture sector, for example, antimicrobial medicines (antibiotics, antiprotozoals and parasiticides) and hormones. The overuse of pharmaceuticals in industrial farming results in a significant release of their residues into soil, groundwater and surface water. The main entry pathways are from the use of animal manure as a fertiliser, animal waste storage and disposal tanks (FAO, 2018[44]). Moreover, the reuse of biosolids as fertiliser or irrigation of recycled wastewater from WWTPs onto agricultural land will eventually spread human pharmaceuticals to the surrounding environment (FAO, 2018[44]). Over time, residues from these drugs accumulate in the soil or drain into surface and groundwater; where they may also be taken up by plants (Weber et al., 2014[3]; Carter et al., 2014[45]).
The release of oestrogen hormones from livestock is the most significant source of oestrogens to the environment. The discharges of oestrogen from livestock are estimated at 83,000 kg per year in the U.S and EU alone (Adeel et al., 2017[46]). This compares to the annual global discharge of oestrogens from oral contraceptives, estimated to be approximately 30,000 kg of natural steroidal oestrogens (E1,E2,E3) and 700 kg of synthetic oestrogens (EE2) (calculations based on 7 billion people). Oral administration of the synthetic steroid hormone17-α-methyl-testosterone in fish hatcheries to produce mono-sex of certain fish species is commonly practiced in south-east Asia, with potential release of effluents to the surrounding water (Rico et al., 2012[47]).
Knowledge gaps exist regarding the sales and consumption of veterinary pharmaceuticals, thus it is difficult to estimate the total amount used and released to the environment. It is however, recorded that more than 70% of the total volume of all medically-important antibiotics in the United States1 (and over 50% in most countries globally) are sold for use for livestock (Review on Antimicrobial Resistance, 2015[48]). One important reason for the difference between human and livestock antibiotic use is that human use is commonly for treatment of infections, whereas livestock use is commonly for disease prevention and to marginally improve growth rates (Martin, Thottathil and Newman, 2015[49]).
Antibiotics are also used in aquaculture to improve the health status of the cultured organisms, to prevent or treat disease outbreaks, and as a growth promoter. Aquaculture systems are hydrologically connected with the surrounding water, thus a considerable amount of antibiotics (70-80%) may be released to the surrounding water (Review on Antimicrobial Resistance, 2015[48]). Almost 90% of the global aquaculture production takes place in Asia, primarily in tropical and subtropical regions (FAO, 2016[50]), where the use of 36 antibiotics has been documented (Rico et al., 2012[47]).
1.3.3. Concentration patterns
Concentration patterns of pharmaceuticals in the environment can be classified as: i) continuous (e.g. from WWTPs), ii) seasonal (linked with farming practices, seasonal influenza and allergies, flow rates and temperature), iii) and intermittent (linked with rainfall events, stormwater overflow and irrigation patterns).
Effluent from WWTPs is continuously discharged into surface water, with contaminant loads varying due to the number of households and hospitals connected, the level of wastewater treatment, disease outbreaks and stochastic environmental conditions. Data on the occurrence and concentrations of some pharmaceuticals in effluents from WWTPs and in surface waters show that pharmaceutical concentrations fluctuate widely (Table 1.2), most probably due to different pharmaceutical doses applied in various regions and inconsistent efficiency of wastewater treatment (Pal et al., 2010[51]). A range of antibiotics, analgesics, anti-inflammatories, anticonvulsants, beta-blockers and blood lipid modifying agents have been detected in various concentrations in both WWTP effluent and receiving surface waters in North America, Europe, Australia and Asia. Antibiotics are of particular concern with levels in effluent in each of these regions greater than the predicted no effect concentration (PNEC), and high proportions of the parent pharmaceutical compound detected following wastewater treatment (Table 1.2) (Pal et al., 2010[51]). Other reviews report similar results, with the exception of trimethoprim and ibuprofen reported at one higher level of magnitude in wastewater effluent in North America (e.g. (Tran, Reinhard and Gin, 2018[33])).
The majority of studies report peaks of human pharmaceutical concentrations during cold seasons (Lindholm-Lehto et al., 2016[53]) (Singer et al., 2014[54]) (Sun et al., 2014[55]) (Yu, Wu and Chang, 2013[56]) (Kot-Wasik, Jakimska and Śliwka-Kaszyńska, 2016[57]). Seasonal peaks of anti-inflammatories, analgesics and antibiotics in WWTP influent and effluent during cold seasons can be explained by increased usage, but also by reduced WWTP removal capacity (reduced microbial activity of activated sludge) due to cooler temperatures (Sun et al., 2014[55]).
Seasonal or intermittent peaks from WWTPs can also be observed during pandemics or other events when the use of a certain set of pharmaceuticals increase within a short timespan. For instance, (Singer et al., 2014[54]) showed that antivirals, antibiotics and decongestants in WWTP effluents and the River Thames, UK increased during November 2009 - the autumnal peak of the 2009 influenza pandemic. Increased effluent concentrations of antihistamines during the spring can be attributed to the onset of spring allergies (Vatovec et al., 2016[58]). Increased pharmaceutical concentrations may also be observed during high rainfall events when sewage bypasses treatment through combined sewer overflows.
Several studies report increased concentrations of pharmaceuticals in water bodies as a result of reduced river flows. For instance, human pharmaceuticals significantly increased during dry weather conditions in South Wales, UK due to reduced dilution with surface water flows (Kasprzyk-Hordern, Dinsdale and Guwy, 2008[59]). Temporal peak concentrations of pharmaceuticals in surface water, as a result of lower rainfall and therefore reduced dilution of WWTP discharges, have also been seen to affect the concentration patterns of pharmaceuticals in drinking water (Padhye et al., 2014[60]).
Concentration peaks of veterinary pharmaceuticals in water bodies, with origins from soil and agricultural practices, are primarily driven by rainfall and the properties of the receiving water body. Most studies suggest that the soil serves as a reservoir and peak concentrations of pharmaceuticals in water are, in general, associated with overland runoff caused by rain events and soil erosion (Jaimes-Correa, Snow and Bartelt-Hunt, 2015[61]) (Bernot, Smith and Frey, 2013[62]) (Forrest et al., 2011[63]) (Lissemore et al., 2006[64]). In addition, variations in the use of veterinary pharmaceuticals may be driven by agricultural practices and increased usage during certain periods (such as calving or lambing), and timing of manure, slurry and irrigation applications. Pharmaceuticals released during summer are expected to undergo more rapid degradation in the environment due to increased temperature and more intense sunlight (Lindholm-Lehto et al., 2016[53]).
1.3.4. Environmental sinks
The occurrence of certain pharmaceuticals in the environment has been acknowledged for several decades. In the environment, active pharmaceutical ingredients are found in surface waters, groundwater, soil, manure, biota, sediment, drinking water and the food chain (Benotti et al., 2009[65]; Michael et al., 2013[13]; Daughton and Ternes, 1999[66]; de Jongh et al., 2012[67]; Mompelat, Le Bot and Thomas, 2009[68]; Monteiro and Boxall, 2010[10]; Verlicchi, Al Aukidy and Zambello, 2012[15]; Lapworth et al., 2012[11]). Thanks to improved analytical techniques and established laboratories, low-levels of environmental pollutants have increasingly been detected in all regions of the world (Table 1.3). In a global review by aus der Beek et al. (2016[5]), a total of 631 human and veterinary pharmaceuticals (including 127 transformation products) were detected in surface, ground and drinking waters in 71 countries. Table 1.3 represents frequently analysed pharmaceuticals (antibiotics, analgesics, oestrogens and blood lipid modifying agents). It does not provide the full picture of pharmaceutical occurrence since other pharmaceuticals have relatively limited monitoring.
Concentrations of pharmaceuticals in surface freshwater are the most documented, and represent the environmental sink receiving most discharges from WWTP effluent, agriculture and aquaculture. Surface waters, in general, contain higher levels and a greater range of pharmaceuticals in comparison to groundwater bodies (Focazio et al., 2008[69]) (Vulliet and Cren-Olivé, 2011[70]). Countries that use surface water as a source of drinking water tend to have higher concentrations of pharmaceuticals in drinking water in comparison to those using groundwater as a drinking water source (BIO Intelligence Service, 2013[20]). However, groundwater is also reported as an important sink, and under certain conditions, may pose a prolonged threat to drinking water sources due to long groundwater residence times (Lapworth et al., 2012[11]). For instance, a study by Barnes et al. (2008[71]) identified pharmaceuticals and personal care products in 81% of sampled groundwater sites in 18 states of the U.S.
The marine environment is less characterised for its occurrence of pharmaceuticals compared to freshwater (Arpin-Pont et al., 2016[72]) but there is increasing evidence that pharmaceuticals are present in, and have the potential to impact, marine and coastal environments (Gaw, Thomas and Hutchinson, 2014[73]; Fabbri and Franzellitti, 2016[74]). For example, estuarine systems receiving chronic inputs of trace concentrations of the antimicrobial tylosin, as well as other antibiotics, may experience reductions in benthic microalgal biomass and primary productivity (Pinckney et al., 2013[75]). Examples of laboratory reported adverse effects for analgesics on marine organisms include: reduced feeding rates (Solé et al., 2010[76]), impacts on survival (Guler and Ford, 2010[77]), reduced mussel byssus strength (Ericson, Thorsén and Kumblad, 2010[78]), oxidative and neurotoxic effects (Mezzelani et al., 2016[79]), and changes in immune response (Solé et al., 2010[76]; Mezzelani et al., 2016[79]) and biochemical markers (Gonzalez-Rey and Bebianno, 2014[80]).
Monitoring is still lacking in both coverage and frequency in developing economies (Puckowski et al., 2016[81]; Madikizela, Tavengwa and Chimuka, 2017[82]). Where studies have been undertaken, higher concentrations of pharmaceutical pollutants have been found (in comparison to developed nations), which may reflect a lack of wastewater treatment infrastructure (Segura et al., 2015[83]).
1.3.5. Future projections on pharmaceuticals in the environment
In OECD countries, pharmaceutical consumption has rapidly grown over the last decade, owing to aging populations, epidemiological changes (increasing need, ability and expectation to treat ageing-related and chronic diseases), and changes in clinical practice (recommendations of earlier treatment, higher dosages or prolonged treatment) (Belloni, Morgan and Paris, 2016[84]). This trend is expected to continue and is reflected in a projected growth rate of the pharmaceutical industry of 6.5% per year by 2022 (UN Environment, 2019[85]). As an example, the growth prognosis for consumption of prescription pharmaceuticals in Germany is presented in Box 1.2. Urbanisation is another factor compounding risks of pharmaceuticals in the environment; a larger group of ageing people will be discharging via a single wastewater treatment plant.
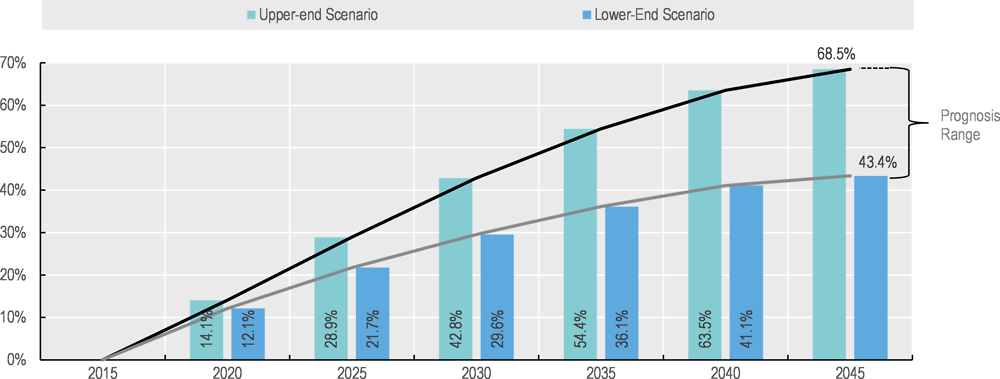
The German Association of Energy and Water Industries (BDEW) investigated the future trends of human pharmaceutical usage in Germany. The prediction was based on a prognostic model of human pharmaceutical usage based on a population projection, differentiated by age and gender-specific consumption. The study concludes that as a result of the demographic change and an increased consumption per capita, pharmaceutical usage is projected to increase by 43-67 percent by the year 2045 (from a baseline of 2015) (Figure 1.3).
Pharmaceutical usage is dominated by older population groups (age 80-85), consuming about 20 times more compared to those of younger age (age 20-25). An ageing population is thought to be the main driver; of the total population, people over the age of 60 years will increase by 9% by 2045. An increase in consumption of pharmaceuticals is also expected in younger age groups (Civity, 2017[86]).
As global meat and fish production is projected to increase2 (Alexandratos and Bruinsma, 2012[87]; OECD/FAO, 2018[88]), as well as demand for companion animal pharmaceuticals (NOAH, 2018[89]), so will the use of veterinary pharmaceuticals increase. For example, antibiotics administered to livestock animals in feed are projected to increase by 67% worldwide by 2030 (from 2015 levels) (Van Boeckel et al., 2015[90]). Much of this increase will come in emerging economies.
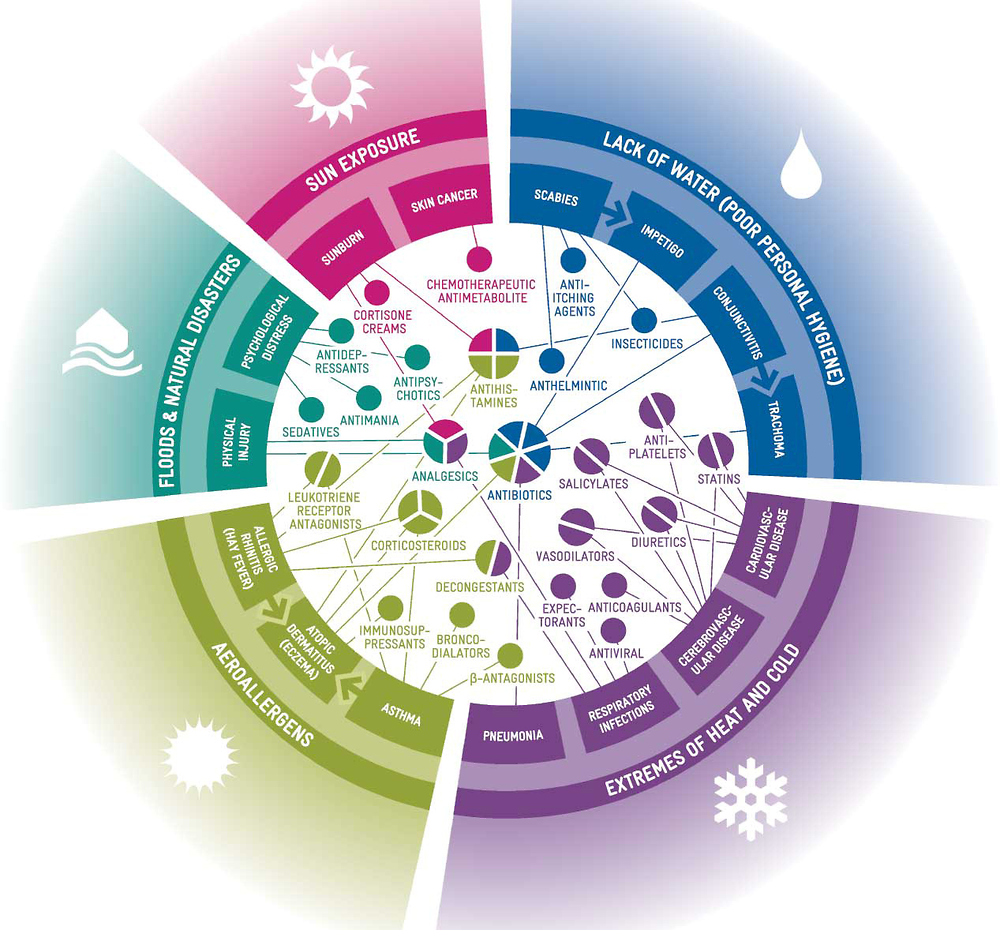
In addition, changes in climate are likely to affect the amounts and types of pharmaceuticals used and released to water bodies. Substantially higher pharmaceutical use appears inevitable as climate change stresses native life, thereby enabling pathogens to cause disease (Cavicchioli et al., 2019[91]). For example, non-communicable diseases (e.g. cardiovascular disease and mental illness) and respiratory, water-borne, vector-borne and food-borne toxicants and infections are expected to become more common (Cavicchioli et al., 2019[91]) (Redshaw et al., 2013[92]) (Figure 1.4). Climate change is predicted to increase the rate of antibiotic resistance of some human pathogens (MacFadden et al., 2018[93]).
Climate-related environmental changes have already been associated with a rise in the incidence of chronic diseases in the Northern Hemisphere (Redshaw et al., 2013[92]). Certain vector-borne diseases, such as bluetongue, an economically important viral disease of livestock, have already emerged in Europe in response to climate change, and larger, more frequent outbreaks are predicted to occur in the future (Jones et al., 2019[94]). Millions of people are predicted to be newly at risk under climate change to mosquito-borne and tick-borne diseases (Cavicchioli et al., 2019[91]).
Climate change will also entail changes in farming practice. For example, increasing extreme weather events will mean livestock populations in some regions will be subjected to thermal stress and waterlogged pastures. This may in turn, lead to increased indoor housing of animals, facilitate the introduction of new pathogens, vectors, and hosts, and thus lead to increased use of veterinary pharmaceuticals (Boxall et al., 2009[95]).
Overall, it is anticipated that climate change will affect the fate and transport of pharmaceuticals, and result in an increase in the likelihood of pharmaceuticals entering the environment. Increases in temperature and changes in moisture content are likely to reduce the persistence of pharmaceuticals (i.e. increase degradation rates) but changes in hydrological characteristics are likely to increase the potential for contaminants to be transported to water bodies. For example, extreme weather events will mobilise contaminants from soils and manure in agriculture systems, potentially increasing their bioavailability, and heavy rainfall events will trigger stormwater overflows, bypassing WWTPs (Boxall, 2012[17]). Conversely, in areas affected by lower rainfall and increased water scarcity, the dilution potential of pharmaceutical residues and metabolites in surface water will be reduced.
As a result of the above projected trends, unless adequate measures are taken to manage the related risks, pharmaceuticals will increasingly be released into the environment (Weber et al., 2014[3]).
copy the linklink copied!1.4. Effects of pharmaceuticals in the environment on human and freshwater ecosystem health
1.4.1. Proven and potential adverse effects and costs
It is challenging to assess the potential long-term risks of trace amounts of pharmaceuticals in the environment, especially given that water resources (including drinking water) are often not systematically monitored for pharmaceutical residues, and their cause and effect. While our understanding of the environmental impact for several pharmaceuticals is increasing3 (Donnachie, Johnson and Sumpter, 2016[96]), the vast majority of pharmaceuticals have not been evaluated for their long-term toxicity, occurrence or fate in the environment, and it is therefore difficult to generalise the risk they may give rise to. Furthermore, whilst pharmaceuticals are stringently regulated for efficacy and patient safety, the adverse effects they may have in the natural environment have not yet been sufficiently studied and are not covered by an international agreement or arrangement (Weber et al., 2014[3]).
Little evidence of risk to human health exists. Currently, quantitative risk assessment studies indicate no appreciable human health risks associated with exposure to pharmaceuticals in drinking water (WHO, 2012[2]). Concentrations of pharmaceuticals found in drinking water are generally greater than 1000-fold below the minimum therapeutic dose, which is the lowest clinically active dosage. Likewise, other environmental risk assessments of human exposure of pharmaceuticals suggests no appreciable risk to human health e.g. (Silva et al., 2017[97]) (Bercu et al., 2008[98]) (Cunningham, Binks and Olson, 2009[99]) (Johnson et al., 2008[100]) (Kostich, Batt and Lazorchak, 2014[101]) (Baken et al., 2018[102]) (Houtman et al., 2014[103]). However, it is important to note that uncertainties and particular concerns still exist. The minimum therapeutic dose for a certain beneficial effect of a pharmaceutical to occur is not related to the effect concentration of other, unintended effects. Some cytotoxic drugs used in anti-cancer treatment will not have a safe lower level if they interact directly with DNA. Many researchers stress the lack of knowledge regarding long-term and low-level exposure of pharmaceutical mixtures in the environment. In most studies, the targeted population consists of healthy adults. But for sensitive populations, such as children, pregnant women, foetuses, and people with allergies and chronic diseases, the risk could be greater (Collier, 2007[104]; Snyder et al., 2008[105]; Johnson et al., 2008[106]; BIO Intelligence Service, 2013[107]). Furthermore, most studies also assume the risk posed by a single compound is comparable to one posed by a mixture (Kümmerer, 2009[9]). Little evidence exists on the potential human health risks from consumption of food containing APIs, although there is evidence proving the potential for bioaccumulation and transfer of APIs through the food web (see section 1.4.2).
Nevertheless, the presence of pharmaceuticals in the environment has raised concerns among drinking water regulators, governments, water suppliers and the public, regarding the proven and potential risks to human and environmental health. Certain pharmaceuticals have been proven to cause undesired adverse effects on ecosystems, including mortality, and changes to physiology, behaviour, reproduction. For example, diclofenac in the environment has resulted in the endangerment of species of vultures, antidepressants have been shown to alter fish behaviour (Box 1.3) and endocrine disrupting pharmaceuticals can interfere with fish reproduction (Box 1.4) (BIO Intelligence Service, 2013[107]; Santos et al., 2010[108]; Oaks et al., 2004[109]; Green et al., 2004[110]). The German Environment Agency (UBA) estimate that 10% of pharmaceutical products indicate a potential environmental risk (Küster and Adler, 2014[111]). Of greatest concern are hormones, antibiotics, analgesics, antidepressants and anticancer pharmaceuticals used for human health, and hormones, antibiotics and parasiticides used as veterinary pharmaceuticals (Küster and Adler, 2014[111]).
In a study by Gunnarsson et al. (2019[112]),, environmental risks of a range of pharmaceuticals with a full set of ecotoxicity data (excluding antibiotics) were assessed based on a combined analysis of drug toxicity and predicted environmental concentrations based on European patient consumption data. Pharmaceuticals that target the endocrine system represented the highest potency and greatest environmental risk (PEC/PNEC >10). Propranolol (beta blocker) and fluoxetine (antidepressant) were found to be of moderate environmental risk (PEC/PNEC >1). Most drugs (> 80%) with a full set of ecotoxicity data indicated a low environmental risks for the endpoints assessed in a European context.
A summary of potential adverse effects of some pharmaceuticals in the environment on freshwater ecosystem and human health is presented Table 1.4.
A growing number of studies link exposure to antidepressants to behavioural alterations in fish, including changes in feeding rate, mating success, parental care, predator avoidance, aggression, reduced social interaction, foraging efficiency, dispersal and migration. These disrupted ecological interactions may affect the food web structure and functions of the ecosystem (Brodin et al., 2014[113]). For instance, Kellner et al. (2016[114]) showed in laboratory experiments that three-spine stickleback fish exposed to the antidepressant citalopram increased their locomotor activity and decreased bottom dwelling. The fish became bolder and less sensitive to stress, which made them more vulnerable to predators.
Sources: (Brodin et al., 2014[113]); (Kellner et al., 2016[114]).
Natural hormones such as oestrogen and progestin are essential for the function of the female reproductive system, while androgens regulate the male sex organs (WHO, 2012[115]). There are natural and synthetic hormones typically found in contraceptives, hormonal therapies and veterinary medicine (as growth-regulators of farmed animals) which are intended to interfere with the natural hormonal system of the body, but when released to the aquatic environment they pose a threat to organisms and their reproduction systems. Other pharmaceuticals that have showed potential to act as endocrine active substances include triclosan (antibacterial and antifungal), fluoxetine (antidepressants) and diclofenac (anti-inflammatory).
Drugs targeting the endocrine system may represent the highest environmental potency and risk (Gunnarsson et al., 2019[112]). By way of example, the effects of the synthetic oestrogen EE2 at concentrations of 5-6 ng/L were demonstrated in a 7-year whole-lake experiment conducted in north-western Ontario, Canada. Male fish (Fathead minnow) in this study underwent feminisation, and females showed delayed ovarian development, leading to the collapse of the fish population (Kidd et al., 2007[116]). Similarly, the progestin levonorgestrel (LNG) has shown to be a highly efficient androgen in female fish. Under laboratory conditions, LNG blocked seasonal inhibition of sperm production in male fish and gave rise to a male-biased sex ratio. The latter effect was seen at a concentration of 10 ng/L, resulting in a population consisting only of male fish (Svensson et al., 2014[117]; Svensson et al., 2013[118]; Svensson et al., 2016[119]). Furthermore, laboratory exposure of LNG or EE2 to frogs have demonstrated that females lacked, or had underdeveloped, oviducts, which caused sterility (Kvarnryd et al., 2011[120]; Gyllenhammar et al., 2009[121]).
The release of hormones may also have unintended adverse effects to humans via exposure through food and drinking water. For instance, oestrogens are believed to give rise to increased risk to breast cancer in females (Moore et al., 2016[122]) and prostate cancer in men (Nelles, Hu and Prins, 2011[123]). Pregnant women, foetuses and infants are particularly at risk if continuously exposed to endocrine disruptors even at low concentrations. Potential diseases linked to endocrine disrupting chemicals include those related to the following systems: reproductive and endocrine (e.g. breast or prostate cancer, infertility, diabetes, early puberty), immune and autoimmune, cardiopulmonary (e.g. asthma or heart disease), and nervous systems (e.g. Alzheimer’s disease, Parkinson’s disease and attention deficit hyperactivity disorder) (WHO, 2012[115]).
Sources: (WHO, 2012[115]) (Kidd et al., 2007[116]) (Svensson et al., 2014[117]; Svensson et al., 2013[118]; Svensson et al., 2016[119]) (Kvarnryd et al., 2011[120]; Gyllenhammar et al., 2009[121]) (Moore et al., 2016[122]) (Nelles, Hu and Prins, 2011[123]) (Godfray et al., 2019[124]) (Gunnarsson et al., 2019[112]).
Economic costs are not always easily defined when it comes to the loss of biodiversity and ecosystem services caused by pharmaceuticals in the environment. However, two examples of adverse effects from diclofenac and ivermectin illustrate the costs can be substantial (see Box 1.5 and Box 1.6 respectively).
Diclofenac is a non-steroidal anti-inflammatory pharmaceutical used in human and veterinary medicine. It was found to be responsible for the >90% decline in the population of three vulture species across the Indian subcontinent in the 1990s and 2000s. The birds suffered from renal failure after feeding on dead cattle previously treated with diclofenac (Green et al., 2004[159]).
As vultures are a keystone species, their population collapse has had a range of ecological, socio-economic, cultural and human impacts. Vultures have historically played an important role in environmental health, by disposing of animal and human remains. As a consequence of the loss of vultures, the dog population in India increased, which was assumed to cause increased loss of human life and health costs; dogs are the main source of rabies. The estimated medical expenses from rabid dog bites was estimated at USD 34 billion over a 14 year period (Markandya et al., 2008[160]).
In 2006, India, Pakistan and Nepal banned the veterinary usage of diclofenac, and introduced a drug called meloxicam as a substitute, to prevent further decline in vulture populations. Both the European Union and the US Environmental Protection Agency have identified diclofenac as an environmental threat. Of note, diclofenac, in this case, was not transited by water, and as such, end-of-pipe measures (e.g. wastewater treatment plant upgrades) would not have an impact on the survival of vulture populations.
Since the ban, of the three species, the White-rumped Vulture population has increased, but the Indian Vulture population continues to decline and the population size of the Slender-billed Vultures is too small to estimate a reliable trend. The main causes are thought to be the re-purposing of diclofenac for human use for livestock use, and the veterinary use of five other non-steroidal anti-inflammatory drugs (aceclofenac, carprofen, flunixin, ketoprofen and nimesulide) that are known to also be toxic to vultures (Prakash et al., 2019[161]).
Sources: (Green et al., 2004[159]) (Markandya et al., 2008[160]) (Prakash et al., 2019[161]).
Ivermectin is a parasiticide used in veterinary medicine. Risk assessments suggest risks to aquatic and terrestrial biota. The dung beetle is one of the most sensitive organisms affected by ivermectin (Liebig et al., 2010[162]). The critical ecological roles of the dung beetle include decomposition of animal waste, recycling nitrogen and control of pest habitats, with significant economic value for the cattle industry (Losey and Vaughan, 2006[163]). In the U.S., the cost of loss in ecosystem services of the dung beetle due to ivermectin in the environment was estimated at USD 380 million per year (Losey and Vaughan, 2006[163]).
Sources: (Liebig et al., 2010[162]) (Losey and Vaughan, 2006[163]).
Antibiotics are a specific group of APIs of potential ecological and human health concern for three reasons: i) all major nutrient cycles in the environment depend on the activity of bacterial communities; ii) antibiotics entering WWTPs may affect bacterial communities used for biological degradation and consequently decrease their efficiency to remove other pollutants from the water (e.g. (Schmidt, Winter and Gallert, 2012[164])) and iii) the mis- and over-use of antibiotics is an important contributing factor of antimicrobial resistance (AMR); up to 50% of the antibiotics prescribed4 for human use are considered unnecessary (Holmes et al., 2017[165]); the number is even greater in the agriculture and aquaculture sectors where in some countries they are administered as a growth promoter and as substitute for good hygiene (Van Boeckel, 2017[18]).
Antimicrobial resistance (AMR) is a global health crisis with the potential for enormous health and economic consequences. An AMR review commissioned by the UK Prime Minister estimates that drug resistant infections cause up to 700,000 deaths each year globally, and will, if no action is undertaken, increase to 10 million per year by 2050 (Review on Antimicrobial Resistance, 2015[48]). In OECD countries, dealing with AMR complications is projected to cost up to USD 3.5 billion every year between 2015 and 2050, which corresponds to 10% of health care costs caused by communicable diseases (OECD, 2018[166]). Box 1.7 provides a brief introduction to AMR, including sources, pathways and potential the adverse effects. For more information on AMR, see OECD (2018[166]), Stemming the Superbug Tide.
Antimicrobial resistance (AMR) is the ability of a microbe to resist the effects of medication that could once successfully destroy or inhibit the microbe. In other words, AMR occurs when microorganisms become resistant to antibiotics, antifungals or antivirals. This phenomenon poses severe risks to global health, livelihoods and food security. Drug resistant infections are estimated to cause 700,000 deaths each year globally (IACG, 2018[167]). To put this in perspective, this is higher than the number of people dying from cancer worldwide, and may also be an underestimation because secondary effects such as avoiding surgery, were not taken into account. In Europe, North America and Australia alone, superbug infections could cost the lives of around 2.4 million over the next 30 years unless more is done to combat AMR (OECD, 2018[166]).
A continued rise in AMR is projected to lead to a reduction of 2-3.5% in Gross Domestic Product globally, with a cumulative cost of up to USD 100 trillion (Review on Antimicrobial Resistance, 2015[48]). In OECD countries, dealing with AMR complications is projected to cost up to USD 3.5 billion every year between 2015 and 2050, which corresponds to 10% of health care costs caused by communicable diseases (OECD, 2018[166]).
AMR is acquired through mutation of existing DNA or by direct exchange from other bacteria. Resistance is not a new phenomenon, bacteria have always evolved so that they can resist drugs. However, due to the misuse and overuse of antibiotics in human and veterinary medicine, AMR has increasingly become a problem as new resistance mechanisms and multi-resistant bacteria rapidly increase worldwide. Antibiotic resistance can spread across national borders via human travellers and food trade, but also via wild birds and animals (Bengtsson-Palme, Kristiansson and Larsson, 2018[168]) (Johnning et al., 2015[169]), making this a global issue.
Resistance is already high, and projected to grow even more rapidly, in low and middle-income countries. In Brazil, Indonesia and Russia, for example, between 40% and 60% of infections are already resistant, compared to an average of 17% in OECD countries. In these countries, growth of AMR rates is forecast to be 4 to 7 times higher than in OECD countries between now and 2050 (OECD, 2018[166]).
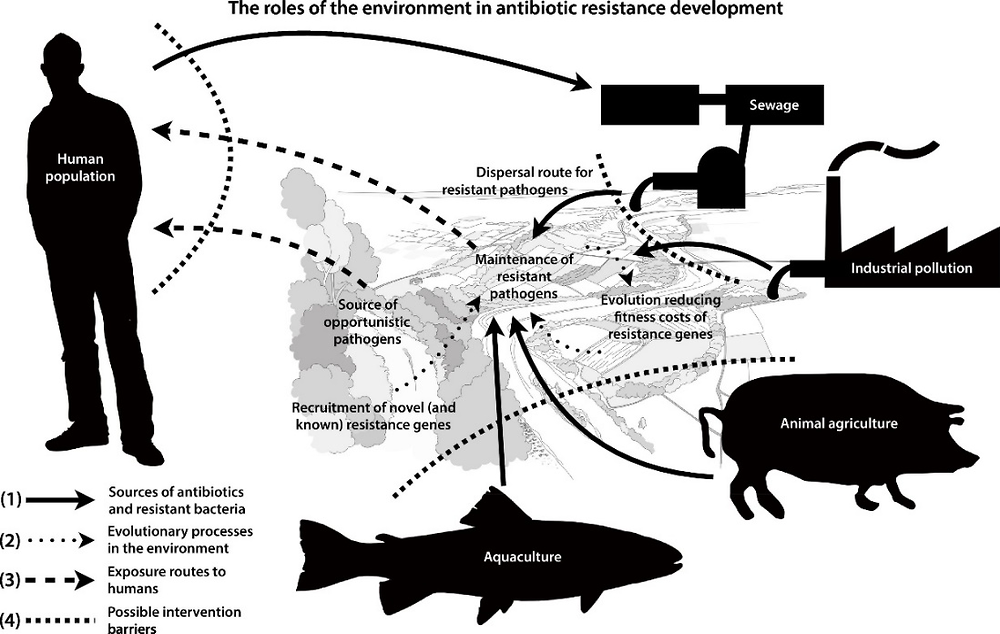
The main sources of antibiotics and AMR bacteria, evolutionary processes in the environment and exposure routes to humans are illustrated in Figure 1.5. The use of antibiotics in human health, agriculture and aquaculture practices causes releases to the environment, which itself is a vector of AMR (IACG, 2018[167]). The role of the environment in the evolution of AMR can be categorised into two processes: i) transmission of resistant genes and/or pathogens, often via faecal contamination of water (Karkman, Pärnänen and Larsson, 2019[170]); and ii) exposure of environmental bacteria to antibiotics which may develop novel (i.e. new) resistance genes which can then be transferred to pathogens (MistraPharma, 2016[171]). Taken together, the environment becomes a reservoir for resistant genes as well as an arena for the development and spread of resistance to pathogens. Many of the resistant genes that are encountered in pathogens today originate from bacteria normally thriving in the environment. However, there is still a lack of knowledge regarding how, and under what circumstances, the environment contributes to the development of AMR (Larsson et al., 2018[172]).
Freshwater ecosystems provide an ideal setting for the acquisition and spread of AMR due to the continuous pollution by antimicrobial compounds derived from anthropogenic activities. Humans can be considered as a source of both antibiotics and antibiotic resistance genes, which may be released into the environment via WWTPs. Antibiotics are also used in veterinary practices, animal husbandry, agriculture and aquaculture.
1.4.2. Pharmaceutical characteristics that affect environmental risks
It is important to not only measure the environmental concentrations of pollutants, but also whether they are toxicologically significant or insignificant, that is, whether they have the potential to cause adverse effects to human or ecosystem health at concentrations observed in the environment. When assessing the risks of pollutants in the environment, there are a number of properties of particular interest, including persistence, bioaccumulation, toxicity and mobility (Schwarzenbach et al., 2006[173]). These properties are briefly explained in the following sections.
Persistence
Persistence refers to chemical substances that withstand natural decomposition, such as biodegradation, hydrolysis or photolysis. Persistent substances increase the potential for long-term and varied effects, and a longer exposure time increases the potential for multiple contamination of the ecosystem (Kümmerer, 2009[9]). Persistent substances remain in their original form in aquatic systems for long periods of time, sometimes affecting water bodies hundreds or thousands of kilometres away from the contaminant source (Schwarzenbach et al., 2006[173]), and therefore potentially turning a pollution source into an international problem (Metz and Ingold, 2014[174]).
Pharmaceuticals that are designed to be slowly degradable, or even non-degradable, present a special risk when they enter, disseminate and persist in the environment. Such substances are referred to as ‘environmentally persistent pharmaceutical pollutants (EPPPs)’ (SAICM, 2015[175]). An example of an EPPP is oxazepam (see Box 1.8). Some transformation products are also hazardous (Godoy and Kummrow, 2017[33]; Česen et al., 2016[47]). In addition, there are ‘pseudo-persistent’ pharmaceutical pollutants, which are degradable, but continuous emissions into the environment can lead to their constant environmental presence (Daughton, 2002[176]). For example, paracetamol and ibuprofen are low persistent pharmaceuticals, but effectively behave as persistent compounds because of their continuous emission to the environment (e.g. via WWTPs), at rates faster than environmental removal (degradation) (Löffler et al., 2005[177]).
Oxazepam was introduced to the pharmaceutical market in the late 1960s and is used to treat anxiety. The persistence of oxazepam in sediment was investigated in a Swedish lake (Lake Ekoln) receiving wastewater effluent from the city of Uppsala. Sediment sampling in the lake in 2013 confirmed that oxazepam used in the early 1970s remained in the sediment, despite in situ degradation processes and sediment diagenesis. The presence of oxazepam suggests that this drug can remain bioactive in sediments for several decades. It was concluded that the persistent properties of oxazepam in sediment were comparable to other chemical contaminants identified as highly persistent.
In the same study, laboratory and field experiments were undertaken on oxazepam in surface water. Results showed that therapeutic forms of oxazepam can persist over several months in cold (<5 °C) lake water free from UV light. The key factors controlling the initial degradation of oxazepam in natural waters were solar UV light, temperature and the total organic carbon of the sediment.
Source: (Klaminder et al., 2015[178]).
Mobility
Mobility refers to the potential of chemical substances to transport in soil and aquatic systems. Pharmaceuticals with certain characteristics (e.g. high water solubility, polarity and low sorption potential) are expected to be more mobile in soil and may quickly be transported to surface water or groundwater, and vice-versa. A range of factors determining the transportation of pharmaceuticals in soil and water include climate (e.g. rainfall), soil pH and the persistent properties of the pharmaceutical in question (Boxall, 2012[179]).
Bioaccumulation
Bioaccumulation refers to a compound that is incorporated into living tissue without being properly excreted or degraded within that living system. Hence, bioaccumulating substances remain in the organism, and in some cases, can also biomagnify through the food chain, meaning that the concentration increases with increasing trophic level.
Knowledge regarding pharmaceuticals in biota and bioaccumulation throughout the food web is scarce (Miller et al., 2018[180]). Reported bioaccumulation is in general higher for invertebrates compared to fish (Miller et al., 2018[180]). Differences in bioaccumulation across species may be attributed to several factors, such as lipid content of organisms, body size, life stage and respiration strategy (Miller et al., 2018[180]), but also pharmacokinetic and pharmacodynamics parameters5 (e.g. transport system, protein binding, metabolism, partitioning into organs and tissues) (Fick et al., 2010[181]). Box 1.9 provides an example of bioaccumulation of three pharmaceuticals at multiple trophic levels in an aquatic food web.
The uptake of five pharmaceuticals (diphenhydramine, oxazepam, trimethoprim, diclofenac and hydroxyzine) was investigated in a field study using an aquatic food-web consisting of fish (European perch) and four aquatic invertebrates (damselfly, mayfly, water louse and Ramshorn snail). The results showed that bioaccumulation of the different pharmaceuticals were species- and substance-specific.
There was no evidence that Diclofenac or Trimethoprim accumulated in the organisms (i.e. they were not detected). In contrast, diphenhydramine, oxazepam and hydroxyzine were detected in all species, with hydroxyzine generally being detected in the highest concentrations.
Mayfly larvae showed a four-fold increase of diphenhydramine, oxazepam and hydroxyzine approximately 30 days after the pharmaceuticals were added. The European perch showed an increased concentration during the study period, even though the water concentration decreased. These findings suggest that pharmaceuticals can remain bioavailable for aquatic organisms for long periods and possibly re-enter the food web at a later phase. This study demonstrates exposure and bioaccumulation; ecological effects were not part of scope. In particular, field-based experiments are needed for assessing true exposure and potential effects in natural systems.
Source: (Lagesson et al., 2016[182]).
Toxicity
Toxicity refers to the property of substances that poses a significant hazard to humans or ecosystems. Toxicity to aquatic organisms is determined by standard acute (i.e. mortality) or chronic (e.g. impaired reproduction and growth) endpoints. Acute effects are observed at concentrations orders of magnitudes higher than measured environmental concentrations, and are thus less likely to occur (Boxall, 2012[17]). Chronic effects to aquatic organisms reported for certain pharmaceuticals include growth inhibition, immobilisation, genotoxicity, reproduction toxicity, neurotoxicity and oxidative stress (see section 1.4.1 and Table 1.4).
When considering toxicity risks, there are a number of factors that can complicate assessments:
Mixture effects. In real life, substances are not isolated in the environment; instead they occur mixed together and in combination with other contaminants. Even if a given substance is at a concentration too low to be harmful (i.e. below the no-observed effect concentration), when mixed in water with other chemicals, research has shown that the combined effect can be harmful (Kortenkamp et al., 2007[183]; Backhaus, 2014[184]; Godoy and Kummrow, 2017[185]). There is growing evidence that mixtures of pharmaceuticals possess a joint toxicity greater (i.e. additive effects) than individual toxicities (e.g. (Kortenkamp, Backhaus and Faust, 2009[186]).
Additive effects. One type of mixture effects are additive effects; the others are antagonistic (combined effect of the mixture smaller than the sum of individual effects) or synergistic (combined effect of the mixture larger than the sum of individual effects). Current empirical knowledge suggests that pharmaceuticals that share the same mode of action (molecular targets) can act additively. For instance, the effect of three beta blockers (Propranolol, Atenolol and Metoprolol) was 10-fold more toxic to algae in terms of photosynthesis inhibition (Escher et al., 2006[187]). Furthermore, additive toxicity of mixtures of selective serotonin reuptake inhibitors (antidepressants, such as Fluoxetine, Paroxetine, Sertraline, and Citalopram) towards aquatic invertebrates and algae has also been demonstrated (Christensen et al., 2007[188]). The progestin’s LNG and norethindrone, in combination with progesterone (a natural hormone), have been shown to inhibit egg development in frogs likely acting additively, at concentrations of 1-10 ng/L, which is notable since the therapeutic plasma concentration in women is more than 1000 times higher (Säfholm et al., 2012[189]; Säfholm et al., 2015[190]). Other additive mixture effects have been documented for quinolone antibiotics (Backhaus, Scholze and Grimme, 2000[191]), oestrogens (Brian et al., 2007[192]), anti-inflammatory drugs (Cleuvers, 2003[193]) and anticancer drugs (Elersek et al., 2016[194]). In addition, there is evidence that pharmaceuticals not sharing the same mode of action (i.e. belonging to different therapeutic groups with different molecular targets) can also have additive effects (Runnalls et al., 2015[195]) (Thrupp et al., 2018[196]) (Neale, Leusch and Escher, 2017[197]) (Yamagishi, Horie and Tatarazako, 2017[198]).
Effects of metabolites and transformation products. In the environment, transformation and degradation reactions alter the mobility, persistence and fate of the pharmaceutical residues (Kümmerer, 2009[9]) (Weber et al., 2014[3]). Metabolites and transformation products represent a large amount of the total pharmaceuticals load. This is of concern since these compounds are rarely evaluated for their toxicity, occurrence or fate, and some evidence suggests that the ecotoxicity of some degradation products can have higher toxicity effects than that of their parent compounds (Godoy and Kummrow, 2017[185]). For example, the transformation products of ranitidine (a histamine) and of Naproxen (a nonsteroidal anti-inflammatory) have been found to be more toxic than the parent compounds, both for acute and chronic values (Isidori et al., 2009[199]; Isidori et al., 2005[200]).. In the Netherlands, the measured presence of transformation products with similar pharmacological activities and concentration levels as their parent compounds illustrates the relevance of monitoring transformation products (and metabolites), and including these in environmental risk assessments (de Jongh et al., 2012[67]).
Multiple routes of exposure. Ecosystems and humans may be continuously exposed via a number of pathways (see section 1.3.2) of low-dose mixtures that can have additive effects.
The main challenges faced by regulators is how to determine the degree to which humans and ecosystems are exposed to pharmaceuticals, what interactions may occur, and what specific human and ecosystem health impacts are associated with chemical mixtures (WHO, 2017[201]). Given the large number of pharmaceuticals on the market, and possible mixture combinations (with other pharmaceuticals and other chemicals), exposure pathways, additive effects and transformation products, it can easily be said that assessing the risk of all possible scenarios would not be feasible. Chapter 2 will consider various monitoring techniques and strategies to assess potential risks.
References
[46] Adeel, M. et al. (2017), “Environmental impact of estrogens on human, animal and plant life: A critical review”, Environment International, Vol. 99, pp. 107-119, https://doi.org/10.1016/j.envint.2016.12.010.
[87] Alexandratos, N. and J. Bruinsma (2012), World Agriculture towards 2030/2050: the 2012 revision, Food and Agriculture Organization of the United Nations, http://www.fao.org/economic/esa.
[135] Araújo, A. et al. (2019), “Anti-cancer drugs in aquatic environment can cause cancer: Insight about mutagenicity in tadpoles”, Science of The Total Environment, Vol. 650, pp. 2284-2293, https://doi.org/10.1016/J.SCITOTENV.2018.09.373.
[151] Armstrong, B. et al. (2016), “Reproductive effects in fathead minnows (Pimphales promelas) following a 21 d exposure to 17α-ethinylestradiol”, Chemosphere, Vol. 144, pp. 366-373, https://doi.org/10.1016/j.chemosphere.2015.08.078.
[8] Arnold, K. et al. (2014), “Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/RSTB.2013.0569.
[72] Arpin-Pont, L. et al. (2016), “Occurrence of PPCPs in the marine environment: a review”, Environmental Science and Pollution Research, Vol. 23/6, pp. 4978-4991, https://doi.org/10.1007/s11356-014-3617-x.
[5] aus der Beek, T. et al. (2016), “Pharmaceuticals in the environment-Global occurrences and perspectives”, Environmental Toxicology and Chemistry, Vol. 35/4, pp. 823-835, https://doi.org/10.1002/etc.3339.
[28] Azuma, T. et al. (2016), “Detection of pharmaceuticals and phytochemicals together with their metabolites in hospital effluents in Japan, and their contribution to sewage treatment plant influents”, Science of the Total Environment, Vol. 548-549, pp. 189-197, https://doi.org/10.1016/j.scitotenv.2015.12.157.
[184] Backhaus, T. (2014), “Medicines, shaken and stirred: A critical review on the ecotoxicology of pharmaceutical mixtures”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/rstb.2013.0585.
[191] Backhaus, T., M. Scholze and L. Grimme (2000), “The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fischeri”, Aquatic Toxicology, Vol. 49/1-2, pp. 49-61, https://doi.org/10.1016/S0166-445X(99)00069-7.
[102] Baken, K. et al. (2018), “Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern”, Environment International, Vol. 118, pp. 293-303, https://doi.org/10.1016/J.ENVINT.2018.05.006.
[41] Barnes, K. et al. (2004), “Pharmaceuticals and other organic waste water contaminants within a leachate plume downgradient of a municipal landfill”, Ground Water Monitoring and Remediation, Vol. 24/2, pp. 119-126, https://doi.org/10.1111/j.1745-6592.2004.tb00720.x.
[71] Barnes, K. et al. (2008), “A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States - I) Groundwater”, Science of the Total Environment, Vol. 402/2-3, pp. 192-200, https://doi.org/10.1016/j.scitotenv.2008.04.028.
[35] Behera, S. et al. (2011), “Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea”, Science of The Total Environment, Vol. 409/20, pp. 4351-4360, https://doi.org/10.1016/J.SCITOTENV.2011.07.015.
[84] Belloni, A., D. Morgan and V. Paris (2016), “Pharmaceutical Expenditure And Policies: Past Trends And Future Challenges”, OECD Health Working Papers, No. 87, OECD Publishing, Paris, https://doi.org/10.1787/5jm0q1f4cdq7-en.
[168] Bengtsson-Palme, J., E. Kristiansson and D. Larsson (2018), “Environmental factors influencing the development and spread of antibiotic resistance”, FEMS Microbiology Reviews, Vol. 42/1, pp. 68-80, https://doi.org/10.1093/femsre/fux053.
[65] Benotti, M. et al. (2009), “Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water”, Environmental Science and Technology, Vol. 43/3, pp. 597-603, https://doi.org/10.1021/es801845a.
[98] Bercu, J. et al. (2008), “Human health risk assessments for three neuropharmaceutical compounds in surface waters”, Regulatory Toxicology and Pharmacology, Vol. 50/3, pp. 420-427, https://doi.org/10.1016/j.yrtph.2008.01.014.
[7] Bernhardt, E., E. Rosi and M. Gessner (2017), “Synthetic chemicals as agents of global change”, Frontiers in Ecology and the Environment, Vol. 15/2, pp. 84-90, https://doi.org/10.1002/fee.1450.
[144] Berninger, J. et al. (2011), “Effects of the antihistamine diphenhydramine on selected aquatic organisms”, Environmental Toxicology and Chemistry, Vol. 30/9, pp. 2065-2072, https://doi.org/10.1002/etc.590.
[62] Bernot, M., L. Smith and J. Frey (2013), “Human and veterinary pharmaceutical abundance and transport in a rural central Indiana stream influenced by confined animal feeding operations (CAFOs)”, Science of the Total Environment, Vol. 445-446, pp. 219-230, https://doi.org/10.1016/j.scitotenv.2012.12.039.
[107] BIO Intelligence Service (2013), Study on the environmental risks of medicinal products.
[20] BIO Intelligence Service (2013), Study on the environmental risks of medicinal products, Final Report prepared for Executive Agency for Health and Consumers, BIO Intelligence Service, Paris, https://ec.europa.eu/health/sites/health/files/files/environment/study_environment.pdf.
[17] Boxall, A. (2012), New and Emerging Water Pollutants arising from Agriculture, OECD, Paris, https://www.oecd.org/tad/sustainable-agriculture/49848768.pdf.
[179] Boxall, A. (2012), New and Emerging Water Pollutants arising from Agriculture, OECD publishing, Paris, https://www.oecd.org/tad/sustainable-agriculture/49848768.pdf.
[43] Boxall, A. (2010), “Veterinary Medicines and the Environment”, Springer, Berlin, Heidelberg, https://doi.org/10.1007/978-3-642-10324-7_12.
[95] Boxall, A. et al. (2009), “Impacts of Climate Change on Indirect Human Exposure to Pathogens and Chemicals from Agriculture”, Environmental Health Perspectives, Vol. 117/4, pp. 508-514, https://doi.org/10.1289/ehp.0800084.
[132] Brain, R. et al. (2008), “Aquatic plants exposed to pharmaceuticals: effects and risks”, Reviews of environmental contamination and toxicology, Vol. 192, pp. 67-115, http://www.ncbi.nlm.nih.gov/pubmed/18020304 (accessed on 27 June 2019).
[192] Brian, J. et al. (2007), “Evidence of estrogenic mixture effects on the reproductive performance of fish”, Environmental Science and Technology, Vol. 41/1, pp. 337-344, https://doi.org/10.1021/es0617439.
[152] Brodin, T. et al. (2013), “Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations”, Science, Vol. 339/6121, pp. 814-815, https://doi.org/10.1126/science.1226850.
[113] Brodin, T. et al. (2014), “Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/rstb.2013.0580.
[1] Burns, E. et al. (2018), Application of prioritization approaches to optimize environmental monitoring and testing of pharmaceuticals, Taylor and Francis Inc., https://doi.org/10.1080/10937404.2018.1465873.
[158] Caldwell, D. et al. (2019), “Environmental risk assessment of metformin and its transformation product guanylurea: II. Occurrence in surface waters of Europe and the United States and derivation of predicted no-effect concentrations”, Chemosphere, Vol. 216, pp. 855-865, https://doi.org/10.1016/J.CHEMOSPHERE.2018.10.038.
[156] Campos, B. et al. (2016), “Depressing Antidepressant: Fluoxetine Affects Serotonin Neurons Causing Adverse Reproductive Responses in Daphnia magna”, Environmental Science and Technology, Vol. 50/11, pp. 6000-6007, https://doi.org/10.1021/acs.est.6b00826.
[45] Carter, L. et al. (2014), “Fate and Uptake of Pharmaceuticals in Soil–Plant Systems”, Journal of Agricultural and Food Chemistry, Vol. 62/4, pp. 816-825, https://doi.org/10.1021/jf404282y.
[91] Cavicchioli, R. et al. (2019), “Scientists’ warning to humanity: microorganisms and climate change”, Nature Reviews Microbiology, p. 1, https://doi.org/10.1038/s41579-019-0222-5.
[133] Česen, M. et al. (2016), “Ecotoxicity and genotoxicity of cyclophosphamide, ifosfamide, their metabolites/transformation products and their mixtures”, Environmental Pollution, Vol. 210, pp. 192-201, https://doi.org/10.1016/j.envpol.2015.12.017.
[188] Christensen, A. et al. (2007), “Mixture and single-substance toxicity of selective serotonin reuptake inhibitors toward algae and crustaceans”, Environmental Toxicology and Chemistry, Vol. 26/1, pp. 85-91, https://doi.org/10.1897/06-219R.1.
[86] Civity (2017), Pharmaceutical usage in the context of demographic change: The significance of growing medication consumption in Germany for raw water resources, Civity Management Consultants.
[193] Cleuvers, M. (2003), “Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects”, Toxicology Letters, Vol. 142/3, pp. 185-194, https://doi.org/10.1016/S0378-4274(03)00068-7.
[104] Collier, A. (2007), “Pharmaceutical Contaminants in Potable Water: Potential Concerns for Pregnant Women and Children”, EcoHealth, Vol. 4/2, pp. 164-171, https://doi.org/10.1007/s10393-007-0105-5.
[37] Comber, S. et al. (2018), “Active pharmaceutical ingredients entering the aquatic environment from wastewater treatment works: A cause for concern?”, Science of The Total Environment, Vol. 613-614, pp. 538-547, https://doi.org/10.1016/J.SCITOTENV.2017.09.101.
[138] Crago, J. et al. (2016), “Age-dependent effects in fathead minnows from the anti-diabetic drug metformin”, General and Comparative Endocrinology, Vol. 232, pp. 185-190, https://doi.org/10.1016/j.ygcen.2015.12.030.
[99] Cunningham, V., S. Binks and M. Olson (2009), “Human health risk assessment from the presence of human pharmaceuticals in the aquatic environment”, Regulatory Toxicology and Pharmacology, Vol. 53/1, pp. 39-45, https://doi.org/10.1016/j.yrtph.2008.10.006.
[176] Daughton, C. (2002), “Environmental stewardship and drugs as pollutants”, The Lancet, Vol. 360/9339, pp. 1035-1036, https://doi.org/10.1016/S0140-6736(02)11176-7.
[30] Daughton, C. and I. Ruhoy (2009), “Environmental footprint of pharmaceuticals: The significance of factors beyond direct excretion to sewers”, Environmental Toxicology and Chemistry, Vol. 28/12, pp. 2495-2521, https://doi.org/10.1897/08-382.1.
[66] Daughton, C. and T. Ternes (1999), “Pharmaceuticals and personal care products in the environment: Agents of subtle change?”, Environmental Health Perspectives, Vol. 107/SUPPL. 6, pp. 907-938, https://doi.org/10.1289/ehp.99107s6907.
[154] De Castro-Català, N. et al. (2017), “Evidence of low dose effects of the antidepressant fluoxetine and the fungicide prochloraz on the behavior of the keystone freshwater invertebrate Gammarus pulex”, Environmental Pollution, Vol. 231, pp. 406-414, https://doi.org/10.1016/j.envpol.2017.07.088.
[67] de Jongh, C. et al. (2012), “Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water”, Science of the Total Environment, Vol. 427-428, pp. 70-77, https://doi.org/10.1016/j.scitotenv.2012.04.010.
[150] de Oliveira, L. et al. (2016), “Acute and chronic ecotoxicological effects of four pharmaceuticals drugs on cladoceran Daphnia magna”, Drug and Chemical Toxicology, Vol. 39/1, pp. 13-21, https://doi.org/10.3109/01480545.2015.1029048.
[36] Deblonde, T., C. Cossu-Leguille and P. Hartemann (2011), “Emerging pollutants in wastewater: A review of the literature”, International Journal of Hygiene and Environmental Health, Vol. 214/6, pp. 442-448, https://doi.org/10.1016/j.ijheh.2011.08.002.
[155] Di Poi, C. et al. (2014), “Cryptic and biochemical responses of young cuttlefish Sepia officinalis exposed to environmentally relevant concentrations of fluoxetine”, Aquatic Toxicology, Vol. 151, pp. 36-45, https://doi.org/10.1016/j.aquatox.2013.12.026.
[96] Donnachie, R., A. Johnson and J. Sumpter (2016), “A rational approach to selecting and ranking some pharmaceuticals of concern for the aquatic environment and their relative importance compared with other chemicals”, Environmental Toxicology and Chemistry, https://doi.org/10.1002/etc.3165.
[136] EC (2016), Fate and effects of cytostatic pharmaceuticals in the environment and the identification of biomarkers for and improved risk assessment on environmental exposure, European Commission, Brussels, https://cordis.europa.eu/project/rcn/96703/brief/en.
[129] Efosa, N. et al. (2017), “Diclofenac can exhibit estrogenic modes of action in male Xenopus laevis, and affects the hypothalamus-pituitary-gonad axis and mating vocalizations”, Chemosphere, Vol. 173, pp. 69-77, https://doi.org/10.1016/j.chemosphere.2017.01.030.
[194] Elersek, T. et al. (2016), “Toxicity of the mixture of selected antineoplastic drugs against aquatic primary producers”, Environmental Science and Pollution Research, Vol. 23/15, pp. 14780-14790, https://doi.org/10.1007/s11356-015-6005-2.
[78] Ericson, H., G. Thorsén and L. Kumblad (2010), “Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels”, Aquatic Toxicology, Vol. 99/2, pp. 223-231, https://doi.org/10.1016/J.AQUATOX.2010.04.017.
[187] Escher, B. et al. (2006), “Comparative ecotoxicological hazard assessment of beta-blockers and their human metabolites using a mode-of-action-based test battery and a QSAR approach”, Environmental Science and Technology, Vol. 40/23, pp. 7402-7408, https://doi.org/10.1021/es052572v.
[202] European Commission (2006), REGULATION (EC) No 1907/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1907&from=EN.
[74] Fabbri, E. and S. Franzellitti (2016), “Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species”, Environmental Toxicology and Chemistry, Vol. 35/4, pp. 799-812, https://doi.org/10.1002/etc.3131.
[44] FAO (2018), More people, more food, worse water? A global review of water pollution from agriculture, Food and Agriculture Organization of the United Nations, Rome, https://reliefweb.int/sites/reliefweb.int/files/resources/CA0146EN.pdf.
[50] FAO (2016), The State of World Fisheries and Aquaculture 2016: Contributing to food security and nutrition for all, Food and Agriculture Organization of the United Nations, Rome.
[139] Ferrari, B. et al. (2003), “Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac”, Ecotoxicology and Environmental Safety, Vol. 55/3, pp. 359-370, https://doi.org/10.1016/S0147-6513(02)00082-9.
[181] Fick, J. et al. (2010), “Predicted critical environmental concentrations for 500 pharmaceuticals”, Regulatory Toxicology and Pharmacology, Vol. 58/3, pp. 516-523, https://doi.org/10.1016/j.yrtph.2010.08.025.
[23] Fick, J. et al. (2009), “Contamination of surface, ground, and drinking water from pharmaceutical production”, Environmental Toxicology and Chemistry, Vol. 28/12, pp. 2522-2527, https://doi.org/10.1897/09-073.1.
[69] Focazio, M. et al. (2008), “A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States - II) Untreated drinking water sources”, Science of the Total Environment, Vol. 402/2-3, pp. 201-216, https://doi.org/10.1016/j.scitotenv.2008.02.021.
[63] Forrest, F. et al. (2011), “A scoping study of livestock antimicrobials in agricultural streams of Alberta”, Canadian Water Resources Journal, Vol. 36/1, pp. 1-16, https://doi.org/10.4296/cwrj3601001.
[38] Gardner, M. et al. (2012), “The significance of hazardous chemicals in wastewater treatment works effluents”, Science of The Total Environment, Vol. 437, pp. 363-372, https://doi.org/10.1016/J.SCITOTENV.2012.07.086.
[148] Garric, J. et al. (2007), “Effects of the parasiticide ivermectin on the cladoceran Daphnia magna and the green alga Pseudokirchneriella subcapitata”, Chemosphere, Vol. 69/6, pp. 903-910, https://doi.org/10.1016/j.chemosphere.2007.05.070.
[73] Gaw, S., K. Thomas and T. Hutchinson (2014), “Sources, impacts and trends of pharmaceuticals in the marine and coastal environment”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, pp. 20130572-20130572, https://doi.org/10.1098/rstb.2013.0572.
[149] Giltrow, E. et al. (2009), “Chronic effects assessment and plasma concentrations of the β-blocker propranolol in fathead minnows (Pimephales promelas)”, Aquatic Toxicology, Vol. 95/3, pp. 195-202, https://doi.org/10.1016/j.aquatox.2009.09.002.
[124] Godfray, H. et al. (2019), “A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife”, Proceedings of the Royal Society B: Biological Sciences, Vol. 286/1897, p. 20182416, https://doi.org/10.1098/rspb.2018.2416.
[185] Godoy, A. and F. Kummrow (2017), “What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review”, Critical Reviews in Environmental Science and Technology, Vol. 47/16, pp. 1453-1496, https://doi.org/10.1080/10643389.2017.1370991.
[80] Gonzalez-Rey, M. and M. Bebianno (2014), “Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis”, Aquatic Toxicology, Vol. 148, pp. 221-230, https://doi.org/10.1016/J.AQUATOX.2014.01.011.
[110] Green, R. et al. (2004), “Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent”, Journal of Applied Ecology, Vol. 41/5, pp. 793-800, https://doi.org/10.1111/j.0021-8901.2004.00954.x.
[159] Green, R. et al. (2004), “Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent”, Journal of Applied Ecology, Vol. 41/5, pp. 793-800, https://doi.org/10.1111/j.0021-8901.2004.00954.x.
[77] Guler, Y. and A. Ford (2010), “Anti-depressants make amphipods see the light”, Aquatic Toxicology, Vol. 99/3, pp. 397-404, https://doi.org/10.1016/J.AQUATOX.2010.05.019.
[112] Gunnarsson, L. et al. (2019), “Pharmacology beyond the patient – The environmental risks of human drugs”, Environment International, Vol. 129, pp. 320-332, https://doi.org/10.1016/J.ENVINT.2019.04.075.
[131] Guo, J., A. Boxall and K. Selby (2015), “Do pharmaceuticals pose a threat to primary producers?”, Critical Reviews in Environmental Science and Technology, Vol. 45/23, https://doi.org/10.1080/10643389.2015.1061873.
[121] Gyllenhammar, I. et al. (2009), “Reproductive toxicity in Xenopus tropicalis after developmental exposure to environmental concentrations of ethynylestradiol”, Aquatic Toxicology, Vol. 91/2, pp. 171-178, https://doi.org/10.1016/j.aquatox.2008.06.019.
[14] Heberer, T. and D. Feldmann (2005), “Contribution of effluents from hospitals and private households to the total loads of diclofenac and carbamazepine in municipal sewage effluents - Modeling versus measurements”, Journal of Hazardous Materials, Vol. 122/3, pp. 211-218, https://doi.org/10.1016/j.jhazmat.2005.03.007.
[34] Hollender, J. et al. (2009), “Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration”, Environmental Science & Technology, Vol. 43/20, pp. 7862-7869, https://doi.org/10.1021/es9014629.
[165] Holmes, D. et al. (2017), “A geospatial approach for identifying and exploring potential naturalwater storage sites”, Water (Switzerland), Vol. 9/8, https://doi.org/10.3390/w9080585.
[103] Houtman, C. et al. (2014), “Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data”, Science of the Total Environment, Vol. 496, pp. 54-62, https://doi.org/10.1016/j.scitotenv.2014.07.022.
[167] IACG (2018), “Future Global Governance for Antimicrobial Resistance IACG Discussion Paper July 2018”, Interagency Coordination Group on Antimicrobial Resistance, IACG, http://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_Future_global_governance_for_AMR_120718.pdf?ua=1.
[200] Isidori, M. et al. (2005), “Ecotoxicity of naproxen and its phototransformation products”, Science of The Total Environment, Vol. 348/1-3, pp. 93-101, https://doi.org/10.1016/J.SCITOTENV.2004.12.068.
[199] Isidori, M. et al. (2009), “Effects of ranitidine and its photoderivatives in the aquatic environment”, Environment International, Vol. 35/5, pp. 821-825, https://doi.org/10.1016/J.ENVINT.2008.12.002.
[61] Jaimes-Correa, J., D. Snow and S. Bartelt-Hunt (2015), “Seasonal occurrence of antibiotics and a beta agonist in an agriculturally-intensive watershed”, Environmental Pollution, Vol. 205, pp. 87-96, https://doi.org/10.1016/j.envpol.2015.05.023.
[169] Johnning, A. et al. (2015), “Quinolone resistance mutations in the faecal microbiota of Swedish travellers to India”, BMC Microbiology, Vol. 15/1, https://doi.org/10.1186/s12866-015-0574-6.
[25] Johnning, A. et al. (2013), “Acquired genetic mechanisms of a multiresistant bacterium isolated from a treatment plant receiving wastewater from antibiotic production”, Applied and Environmental Microbiology, Vol. 79/23, pp. 7256-7263, https://doi.org/10.1128/AEM.02141-13.
[106] Johnson, A. et al. (2008), “Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study”, Journal of Hydrology, Vol. 348/1-2, pp. 167-175, https://doi.org/10.1016/J.JHYDROL.2007.09.054.
[100] Johnson, A. et al. (2008), “Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study”, Journal of Hydrology, Vol. 348/1-2, pp. 167-175, https://doi.org/10.1016/j.jhydrol.2007.09.054.
[94] Jones, A. et al. (2019), “Bluetongue risk under future climates”, Nature Climate Change, Vol. 9/2, pp. 153-157, https://doi.org/10.1038/s41558-018-0376-6.
[146] Jonsson, M. et al. (2014), “Antihistamines and aquatic insects: Bioconcentration and impacts on behavior in damselfly larvae (Zygoptera)”, Science of the Total Environment, Vol. 472, pp. 108-111, https://doi.org/10.1016/j.scitotenv.2013.10.104.
[170] Karkman, A., K. Pärnänen and D. Larsson (2019), “Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments”, Nature Communications, Vol. 10/1, p. 80, https://doi.org/10.1038/s41467-018-07992-3.
[59] Kasprzyk-Hordern, B., R. Dinsdale and A. Guwy (2008), “The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK”, Water Research, Vol. 42/13, pp. 3498-3518, https://doi.org/10.1016/j.watres.2008.04.026.
[114] Kellner, M. et al. (2016), “Waterborne citalopram has anxiolytic effects and increases locomotor activity in the three-spine stickleback (Gasterosteus aculeatus)”, Aquatic Toxicology, Vol. 173, pp. 19-28, https://doi.org/10.1016/j.aquatox.2015.12.026.
[6] Khetan, S. and T. Collins (2007), “Human pharmaceuticals in the aquatic environment: A challenge to green chemisty”, Chemical Reviews, Vol. 107/6, pp. 2319-2364, https://doi.org/10.1021/cr020441w.
[116] Kidd, K. et al. (2007), “Collapse of a fish population after exposure to a synthetic estrogen”, Proceedings of the National Academy of Sciences of the United States of America, Vol. 104/21, pp. 8897-8901, https://doi.org/10.1073/pnas.0609568104.
[178] Klaminder, J. et al. (2015), “Long-Term Persistence of an Anxiolytic Drug (Oxazepam) in a Large Freshwater Lake”, Environmental Science and Technology, Vol. 49/17, pp. 10406-10412, https://doi.org/10.1021/acs.est.5b01968.
[186] Kortenkamp, A., T. Backhaus and M. Faust (2009), State of the Art Report on Mixture Toxicity, The School of Pharmacy, University of London, http://ec.europa.eu/environment/chemicals/effects/pdf/report_mixture_toxicity.pdf.
[183] Kortenkamp, A. et al. (2007), “Low-level exposure to multiple chemicals: Reason for human health concerns?”, Environmental Health Perspectives, Vol. 115/SUPPL1, pp. 106-114, https://doi.org/10.1289/ehp.9358.
[101] Kostich, M., A. Batt and J. Lazorchak (2014), “Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation”, Environmental Pollution, Vol. 184, pp. 354-359, https://doi.org/10.1016/J.ENVPOL.2013.09.013.
[57] Kot-Wasik, A., A. Jakimska and M. Śliwka-Kaszyńska (2016), “Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants”, Environmental Monitoring and Assessment, Vol. 188/12, https://doi.org/10.1007/s10661-016-5637-0.
[22] Kristiansson, E. et al. (2011), “Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements”, PLoS ONE, Vol. 6/2, https://doi.org/10.1371/journal.pone.0017038.
[145] Kristofco, L. et al. (2016), “Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine”, Aquatic Toxicology, Vol. 170, pp. 344-354, https://doi.org/10.1016/J.AQUATOX.2015.09.011.
[9] Kümmerer, K. (2009), “The presence of pharmaceuticals in the environment due to human use – present knowledge and future challenges”, Journal of Environmental Management, Vol. 90/8, pp. 2354-2366, https://doi.org/10.1016/J.JENVMAN.2009.01.023.
[111] Küster, A. and N. Adler (2014), “Pharmaceuticals in the environment: Scientific evidence of risks and its regulation”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/rstb.2013.0587.
[120] Kvarnryd, M. et al. (2011), “Early life progestin exposure causes arrested oocyte development, oviductal agenesis and sterility in adult Xenopus tropicalis frogs”, Aquatic Toxicology, Vol. 103/1-2, pp. 18-24, https://doi.org/10.1016/j.aquatox.2011.02.003.
[29] Lacorte, S. et al. (2018), “Pharmaceuticals released from senior residences: occurrence and risk evaluation”, Environmental Science and Pollution Research, Vol. 25/7, pp. 6095-6106, https://doi.org/10.1007/s11356-017-9755-1.
[182] Lagesson, A. et al. (2016), “Bioaccumulation of five pharmaceuticals at multiple trophic levels in an aquatic food web - Insights from a field experiment”, Science of the Total Environment, Vol. 568, pp. 208-215, https://doi.org/10.1016/j.scitotenv.2016.05.206.
[11] Lapworth, D. et al. (2012), “Emerging organic contaminants in groundwater: A review of sources, fate and occurrence”, Environmental Pollution, Cited By (since 1996):98<br/>Export Date: 20 November 2014, pp. 287-303, http://www.scopus.com/inward/record.url?eid=2-s2.0-84856446159&partnerID=40&md5=3907089c51794cbf32d82f845fbea5b2.
[12] Larsson, D. (2014), “Pollution from drug manufacturing: review and perspectives”, Philosophical transactions of the Royal Society of London. Series B, Biological sciences, Vol. 369/1656, https://doi.org/10.1098/rstb.2013.0571.
[21] Larsson, D. (2014), “Pollution from drug manufacturing: Review and perspectives”, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369/1656, https://doi.org/10.1098/rstb.2013.0571.
[172] Larsson, D. et al. (2018), “Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance”, Environment International, Vol. 117, pp. 132-138, https://doi.org/10.1016/j.envint.2018.04.041.
[24] Larsson, D., C. de Pedro and N. Paxeus (2007), “Effluent from drug manufactures contains extremely high levels of pharmaceuticals”, Journal of Hazardous Materials, Vol. 148/3, pp. 751-755, https://doi.org/10.1016/j.jhazmat.2007.07.008.
[19] Larsson, D., C. de Pedro and N. Paxeus (2007), “Effluent from drug manufactures contains extremely high levels of pharmaceuticals”, Journal of Hazardous Materials, Vol. 148/3, pp. 751-755, https://doi.org/10.1016/j.jhazmat.2007.07.008.
[157] Lazzara, R. et al. (2012), “Low environmental levels of fluoxetine induce spawning and changes in endogenous estradiol levels in the zebra mussel Dreissena polymorpha”, Aquatic Toxicology, Vol. 106-107, pp. 123-130, https://doi.org/10.1016/j.aquatox.2011.11.003.
[162] Liebig, M. et al. (2010), “Environmental risk assessment of ivermectin: A case study”, Integrated Environmental Assessment and Management, Vol. 6/SUPPL. 1, pp. 567-587, https://doi.org/10.1002/ieam.96.
[53] Lindholm-Lehto, P. et al. (2016), “Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland”, Environmental Science and Pollution Research, Vol. 23/8, pp. 7985-7997, https://doi.org/10.1007/s11356-015-5997-y.
[64] Lissemore, L. et al. (2006), “An exposure assessment for selected pharmaceuticals within a watershed in Southern Ontario”, Chemosphere, Vol. 64/5, pp. 717-729, https://doi.org/10.1016/j.chemosphere.2005.11.015.
[177] Löffler, D. et al. (2005), “Environmental Fate of Pharmaceuticals in Water/Sediment Systems”, Environmental Science & Technology, Vol. 39/14, pp. 5209-5218, https://doi.org/10.1021/es0484146.
[163] Losey, J. and M. Vaughan (2006), “The economic value of ecological services provided by insects”, BioScience, Vol. 56/4, pp. 311-323, https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2.
[93] MacFadden, D. et al. (2018), “Antibiotic resistance increases with local temperature”, Nature Climate Change, Vol. 8/6, pp. 510-514, https://doi.org/10.1038/s41558-018-0161-6.
[82] Madikizela, L., N. Tavengwa and L. Chimuka (2017), “Status of pharmaceuticals in African water bodies: Occurrence, removal and analytical methods”, Journal of Environmental Management, Vol. 193, pp. 211-220, https://doi.org/10.1016/j.jenvman.2017.02.022.
[26] Marathe, N. et al. (2013), “A treatment plant receiving waste water from multiple bulk drug manufacturers is a reservoir for highly multi-drug resistant integron-bearing bacteria.”, PloS one, Vol. 8/10, https://doi.org/10.1371/journal.pone.0077310.
[160] Markandya, A. et al. (2008), “Counting the cost of vulture decline-An appraisal of the human health and other benefits of vultures in India”, Ecological Economics, Vol. 67/2, pp. 194-204, https://doi.org/10.1016/j.ecolecon.2008.04.020.
[140] Martinez, C. et al. (2018), “In vivo study of teratogenic and anticonvulsant effects of antiepileptics drugs in zebrafish embryo and larvae”, Neurotoxicology and Teratology, Vol. 66, pp. 17-24, https://doi.org/10.1016/j.ntt.2018.01.008.
[49] Martin, M., S. Thottathil and T. Newman (2015), “Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers.”, American journal of public health, Vol. 105/12, pp. 2409-10, https://doi.org/10.2105/AJPH.2015.302870.
[126] Mathias, F. et al. (2018), “Effects of low concentrations of ibuprofen on freshwater fish Rhamdia quelen”, Environmental Toxicology and Pharmacology, Vol. 59, pp. 105-113, https://doi.org/10.1016/j.etap.2018.03.008.
[147] Meinertz, J. et al. (2010), “Chronic toxicity of diphenhydramine hydrochloride and erythromycin thiocyanate to Daphnia, Daphnia magna, in a continuous exposure test system”, Bulletin of Environmental Contamination and Toxicology, Vol. 85/5, pp. 447-451, https://doi.org/10.1007/s00128-010-0117-7.
[31] Melvin, S. and F. Leusch (2016), “Removal of trace organic contaminants from domestic wastewater: A meta-analysis comparison of sewage treatment technologies”, Environment International, Vol. 92-93, pp. 183-188, https://doi.org/10.1016/J.ENVINT.2016.03.031.
[174] Metz, F. and K. Ingold (2014), “Sustainable wastewater management: Is it possible to regulate micropollution in the future by learning from the past? A policy analysis”, Sustainability (Switzerland), Vol. 6/4, pp. 1992-2012, https://doi.org/10.3390/su6041992.
[128] Mezzelani, M. et al. (2016), “Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: Bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis”, Marine Environmental Research, Vol. 121, pp. 31-39, https://doi.org/10.1016/j.marenvres.2016.03.005.
[79] Mezzelani, M. et al. (2016), “Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: Bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis”, Marine Environmental Research, Vol. 121, pp. 31-39, https://doi.org/10.1016/J.MARENVRES.2016.03.005.
[13] Michael, I. et al. (2013), “Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review”, Water Research, Vol. 47/3, pp. 957-995, https://doi.org/10.1016/j.watres.2012.11.027.
[180] Miller, T. et al. (2018), “A review of the pharmaceutical exposome in aquatic fauna”, Environmental Pollution, https://doi.org/10.1016/j.envpol.2018.04.012.
[171] MistraPharma (2016), Identification and Reduction of Environmental Risks Caused by Human Pharmaceuticals. MistraPharma Research 2008–2015. Final report.
[68] Mompelat, S., B. Le Bot and O. Thomas (2009), “Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water”, Environment International, Vol. 35/5, pp. 803-814, https://doi.org/10.1016/j.envint.2008.10.008.
[10] Monteiro, S. and A. Boxall (2010), Occurrence and Fate of Human Pharmaceuticals in the Environment, https://doi.org/10.1007/978-1-4419-1157-5_2.
[122] Moore, S. et al. (2016), “Endogenous Estrogens, Estrogen Metabolites, and Breast Cancer Risk in Postmenopausal Chinese Women”, Journal of the National Cancer Institute, Vol. 108/10, https://doi.org/10.1093/jnci/djw103.
[4] Mullard, A. (2019), 2018 FDA drug approvals, NLM (Medline), https://doi.org/10.1038/d41573-019-00014-x.
[125] Näslund, J. et al. (2017), “Diclofenac affects kidney histology in the three-spined stickleback (Gasterosteus aculeatus) at low Μg/L concentrations”, Aquatic Toxicology, Vol. 189, pp. 87-96, https://doi.org/10.1016/j.aquatox.2017.05.017.
[197] Neale, P., F. Leusch and B. Escher (2017), “Applying mixture toxicity modelling to predict bacterial bioluminescence inhibition by non-specifically acting pharmaceuticals and specifically acting antibiotics”, Chemosphere, https://doi.org/10.1016/j.chemosphere.2017.01.018.
[123] Nelles, J., W. Hu and G. Prins (2011), “Estrogen action and prostate cancer”, Expert Review of Endocrinology and Metabolism, Vol. 6/3, pp. 437-451, https://doi.org/10.1586/eem.11.20.
[137] Niemuth, N. et al. (2015), “Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish”, Environmental Toxicology and Chemistry, Vol. 34/2, pp. 291-296, https://doi.org/10.1002/etc.2793.
[89] NOAH (2018), Industry Facts and Figures: National Office of Animal Health, https://www.noah.co.uk/about/industry-facts-and-figures/ (accessed on 28 December 2018).
[109] Oaks, J. et al. (2004), “Diclofenac residues as the cause of vulture population decline in Pakistan”, Nature, Vol. 427/6975, pp. 630-633, https://doi.org/10.1038/nature02317.
[166] OECD (2018), Stemming the Superbug Tide: Just A Few Dollars More, OECD Health Policy Studies, OECD Publishing, Paris, https://dx.doi.org/10.1787/9789264307599-en.
[88] OECD/FAO (2018), OECD-FAO Agricultural Outlook 2018-2027, OECD Publishing, Paris/FAO, Rome, https://dx.doi.org/10.1787/agr_outlook-2018-en.
[60] Padhye, L. et al. (2014), “Year-long evaluation on the occurrence and fate ofpharmaceuticals, personal care products, andendocrine disrupting chemicals in an urban drinking water treatment plant”, Water Research, Vol. 51, pp. 266-276, https://doi.org/10.1016/j.watres.2013.10.070.
[51] Pal, A. et al. (2010), “Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects”, Science of The Total Environment, Vol. 408/24, pp. 6062-6069, https://doi.org/10.1016/J.SCITOTENV.2010.09.026.
[75] Pinckney, J. et al. (2013), “Sublethal effects of the antibiotic tylosin on estuarine benthic microalgal communities”, Marine Pollution Bulletin, Vol. 68/1-2, pp. 8-12, https://doi.org/10.1016/J.MARPOLBUL.2013.01.006.
[142] Porsbring, T. et al. (2009), “Toxicity of the pharmaceutical clotrimazole to marine microalgal communities”, Aquatic Toxicology, Vol. 91/3, pp. 203-211, https://doi.org/10.1016/J.AQUATOX.2008.11.003.
[161] Prakash, V. et al. (2019), “Recent changes in populations of Critically Endangered Gyps vultures in India”, Bird Conservation International, Vol. 29/1, pp. 55-70, https://doi.org/10.1017/S0959270917000545.
[42] Product Stewardship Council (2018), Webinar | Global Best Practices for Drug Take-Back Programs - Product Stewardship Institute (PSI), https://www.productstewardship.us/page/20180607_GBPFDTBP (accessed on 23 July 2018).
[81] Puckowski, A. et al. (2016), “Bioaccumulation and analytics of pharmaceutical residues in the environment: A review”, Journal of Pharmaceutical and Biomedical Analysis, Vol. 127, pp. 232-255, https://doi.org/10.1016/j.jpba.2016.02.049.
[92] Redshaw, C. et al. (2013), “Potential Changes in Disease Patterns and Pharmaceutical Use in Response to Climate Change”, Journal of Toxicology and Environmental Health, Part B, Vol. 16/5, pp. 285-320, https://doi.org/10.1080/10937404.2013.802265.
[48] Review on Antimicrobial Resistance (2015), ANTIMICROBIALS IN AGRICULTURE AND THE ENVIRONMENT: REDUCING UNNECESSARY USE AND WASTE.
[47] Rico, A. et al. (2012), “Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: A critical review”, Reviews in Aquaculture, Vol. 4/2, pp. 75-93, https://doi.org/10.1111/j.1753-5131.2012.01062.x.
[130] Roose-Amsaleg, C. and A. Laverman (2016), “Do antibiotics have environmental side-effects? Impact of synthetic antibiotics on biogeochemical processes”, Environmental Science and Pollution Research, Vol. 23/5, pp. 4000-4012, https://doi.org/10.1007/s11356-015-4943-3.
[195] Runnalls, T. et al. (2015), “From single chemicals to mixtures-Reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow”, Aquatic Toxicology, Vol. 169, pp. 152-167, https://doi.org/10.1016/j.aquatox.2015.10.009.
[40] Saad, W. et al. (2017), “Drug product immobilization in recycled polyethylene/polypropylene reclaimed from municipal solid waste: experimental and numerical assessment”, Environmental Technology (United Kingdom), Vol. 38/23, pp. 3064-3073, https://doi.org/10.1080/09593330.2017.1288271.
[190] Säfholm, M. et al. (2015), “Mixture effects of levonorgestrel and ethinylestradiol: Estrogenic biomarkers and hormone receptor mRNA expression during sexual programming”, Aquatic Toxicology, Vol. 161, pp. 146-153, https://doi.org/10.1016/j.aquatox.2015.02.004.
[189] Säfholm, M. et al. (2012), “Disrupted Oogenesis in the Frog Xenopus tropicalis after Exposure to Environmental Progestin Concentrations”, Biology of Reproduction, Vol. 86/4, https://doi.org/10.1095/biolreprod.111.097378.
[175] SAICM (2015), Nomination for new emerging policy issue: environmentally persistent pharmaceutical pollutants, http://www.saicm.org/Portals/12/documents/meetings/ICCM4/doc/K1502367%20SAICM-ICCM4-7-e.pdf (accessed on 23 January 2019).
[108] Santos, L. et al. (2010), “Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment”, Journal of Hazardous Materials, Vol. 175/1-3, pp. 45-95, https://doi.org/10.1016/J.JHAZMAT.2009.10.100.
[164] Schmidt, S., J. Winter and C. Gallert (2012), “Long-Term Effects of Antibiotics on the Elimination of Chemical Oxygen Demand, Nitrification, and Viable Bacteria in Laboratory-Scale Wastewater Treatment Plants”, Archives of Environmental Contamination and Toxicology, Vol. 63/3, pp. 354-364, https://doi.org/10.1007/s00244-012-9773-4.
[153] Schultz, M. et al. (2011), “Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows”, Aquatic Toxicology, Vol. 104/1-2, pp. 38-47, https://doi.org/10.1016/j.aquatox.2011.03.011.
[173] Schwarzenbach, R. et al. (2006), “The challenge of micropollutants in aquatic systems”, Science, Vol. 313/5790, pp. 1072-1077, https://doi.org/10.1126/science.1127291.
[27] Scott, T. et al. (2018), “Pharmaceutical manufacturing facility discharges can substantially increase the pharmaceutical load to U.S. wastewaters”, Science of the Total Environment, Vol. 636, pp. 69-79, https://doi.org/10.1016/j.scitotenv.2018.04.160.
[83] Segura, P. et al. (2015), “Global occurrence of anti-infectives in contaminated surface waters: Impact of income inequality between countries”, Environment International, Vol. 80, pp. 89-97, https://doi.org/10.1016/j.envint.2015.04.001.
[97] Silva, L. et al. (2017), “SSRIs antidepressants in marine mussels from Atlantic coastal areas and human risk assessment”, Science of the Total Environment, Vol. 603-604, pp. 118-125, https://doi.org/10.1016/j.scitotenv.2017.06.076.
[54] Singer, A. et al. (2014), “Intra- and inter-pandemic variations of antiviral, antibiotics and decongestants in wastewater treatment plants and receiving rivers”, PLoS ONE, Vol. 9/9, https://doi.org/10.1371/journal.pone.0108621.
[105] Snyder, S. et al. (2008), Toxicological Relevance of EDCs and Pharmaceuticals in Drinking Water, AWWA Research Foundation, Denver, CO.
[76] Solé, M. et al. (2010), “Effects on feeding rate and biomarker responses of marine mussels experimentally exposed to propranolol and acetaminophen”, Analytical and Bioanalytical Chemistry, Vol. 396/2, pp. 649-656, https://doi.org/10.1007/s00216-009-3182-1.
[143] Stoker, T., E. Gibson and L. Zorrilla (2010), “Triclosan exposure modulates estrogen-dependent responses in the female wistar rat”, Toxicological Sciences, Vol. 117/1, pp. 45-53, https://doi.org/10.1093/toxsci/kfq180.
[55] Sun, Q. et al. (2014), “Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China”, Journal of Hazardous Materials, Vol. 277, pp. 69-75, https://doi.org/10.1016/j.jhazmat.2013.11.056.
[117] Svensson, J. et al. (2014), “Environmental concentrations of an androgenic progestin disrupts the seasonal breeding cycle in male three-spined stickleback (Gasterosteus aculeatus)”, Aquatic Toxicology, Vol. 147, pp. 84-91, https://doi.org/10.1016/j.aquatox.2013.12.013.
[118] Svensson, J. et al. (2013), “The synthetic progestin levonorgestrel is a potent androgen in the three-spined stickleback (Gasterosteus aculeatus)”, Environmental Science and Technology, Vol. 47/4, pp. 2043-2051, https://doi.org/10.1021/es304305k.
[119] Svensson, J. et al. (2016), “Developmental exposure to progestins causes male bias and precocious puberty in zebrafish (Danio rerio)”, Aquatic Toxicology, Vol. 177, pp. 316-323, https://doi.org/10.1016/j.aquatox.2016.06.010.
[196] Thrupp, T. et al. (2018), “The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones”, Science of the Total Environment, Vol. 619-620, pp. 1482-1492, https://doi.org/10.1016/j.scitotenv.2017.11.081.
[39] Tong, A., B. Peake and R. Braund (2011), “Disposal practices for unused medications around the world”, Environment International, Vol. 37/1, pp. 292-298, https://doi.org/10.1016/j.envint.2010.10.002.
[33] Tran, N., M. Reinhard and K. Gin (2018), Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review, https://doi.org/10.1016/j.watres.2017.12.029.
[85] UN Environment (2019), Global Chemicals Outlook II: From legacies to innovative solutions, United Nations Environment Programme, https://wedocs.unep.org/bitstream/handle/20.500.11822/27651/GCOII_synth.pdf?sequence=1&isAllowed=y (accessed on 27 June 2019).
[18] Van Boeckel, T. (2017), A Global Plan To Cut Antimicrobial Use In Animals, https://cddep.org/blog/posts/global-plan-cut-antimicrobial-use-animals/ (accessed on 11 September 2018).
[90] Van Boeckel, T. et al. (2015), “Global trends in antimicrobial use in food animals.”, Proceedings of the National Academy of Sciences of the United States of America, Vol. 112/18, pp. 5649-54, https://doi.org/10.1073/pnas.1503141112.
[58] Vatovec, C. et al. (2016), “Investigating dynamic sources of pharmaceuticals: Demographic and seasonal use are more important than down-the-drain disposal in wastewater effluent in a University City setting”, Science of the Total Environment, Vol. 572, pp. 906-914, https://doi.org/10.1016/j.scitotenv.2016.07.199.
[15] Verlicchi, P., M. Al Aukidy and E. Zambello (2012), “Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review”, Science of the Total Environment, Vol. 429, pp. 123-155, https://doi.org/10.1016/j.scitotenv.2012.04.028.
[16] Verlicchi, P. et al. (2010), “Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options”, Journal of Hydrology, Vol. 389/3-4, pp. 416-428, https://doi.org/10.1016/j.jhydrol.2010.06.005.
[141] Vestel, J. et al. (2016), “Use of acute and chronic ecotoxicity data in environmental risk assessment of pharmaceuticals”, Environmental Toxicology and Chemistry, Vol. 35/5, pp. 1201-1212, https://doi.org/10.1002/etc.3260.
[70] Vulliet, E. and C. Cren-Olivé (2011), “Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption”, Environmental Pollution, Vol. 159/10, pp. 2929-2934, https://doi.org/10.1016/j.envpol.2011.04.033.
[52] Watkinson, A. et al. (2009), “The occurrence of antibiotics in an urban watershed: From wastewater to drinking water”, Science of The Total Environment, Vol. 407/8, pp. 2711-2723, https://doi.org/10.1016/J.SCITOTENV.2008.11.059.
[3] Weber, F. et al. (2014), Pharmaceuticals in the environment - The global perspective: Occurrence, effects, and potential cooperative action under SAICM, German Federal Environmental Agency.
[201] WHO (2017), Chemical mixtures in source water and drinking-water, World Health Organisation, Geneva.
[2] WHO (2012), Pharmaceuticals in Drinking Water, World Health Organisation, Geneva.
[115] WHO (2012), State of the Science of Endocrine Disrupting Chemicals 2012 Summary for Decision-Makers INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS, World Health Organization.
[127] Xia, L., L. Zheng and J. Zhou (2017), “Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio)”, Chemosphere, Vol. 182, pp. 416-425, https://doi.org/10.1016/j.chemosphere.2017.05.054.
[198] Yamagishi, T., Y. Horie and N. Tatarazako (2017), “Synergism between macrolide antibiotics and the azole fungicide ketoconazole in growth inhibition testing of the green alga Pseudokirchneriella subcapitata”, Chemosphere, Vol. 174, pp. 1-7, https://doi.org/10.1016/j.chemosphere.2017.01.071.
[32] Yang, Y. et al. (2017), Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review, https://doi.org/10.1016/j.scitotenv.2017.04.102.
[56] Yu, Y., L. Wu and A. Chang (2013), “Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants”, Science of the Total Environment, Vol. 442, pp. 310-316, https://doi.org/10.1016/j.scitotenv.2012.10.001.
[134] Zounková, R. et al. (2007), “Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals”, Environmental Toxicology and Chemistry, Vol. 26/10, p. 2208, https://doi.org/10.1897/07-137R.1.
Notes
← 1. The total volume of medically important antibiotics sold for use in food animals based on 2014 FDA data was 9,479,339 kg. The total volume of antibiotics sold for use in humans based on IMS Health calculations was 3,494,673 kg. The figures are rounded to 73% used in animals and 27% used in humans.
← 2. Global meat production is projected to be 15% higher in 2027 relative to a base period of 2015-17. Over the same time period, global fish production is expected to increase by 13.4%. World growth in fish production will be completely founded upon the continued growth in aquaculture output; capture fisheries production is expected to fall (OECD/FAO, 2018[88]).
← 3. For example, information is well established and increasing for the following pharmaceuticals: diclofenac, paracetamol, ibuprofen, carbamazepine, naproxen, atenolol, ethinyloestradiol, aspirin, fluoxetine, propranolol, metoprolol and sulfamethoxazole.
← 4. In many developing nations, antibiotics may be purchased over-the-counter, without a doctor’s prescription.
← 5. Pharmacodynamics is the study of how a drug affects an organism. Pharmacokinetics is the study of how an organism affects a drug. Both together influence dosing, benefit, and adverse effects.