Chapter 2. Reproductive biology of the mosquito Ae. aegypti
This chapter details the four life stages of mosquito Aedes aegypti in their reproductive biology aspects. The breeding sites that can be either natural sites or artificial containers provided by human habitats. The eggs can survive dry conditions, their hatching and embryonic development depending on humidity and temperature. The larval and pupal stages are strictly aquatic, the whole development phase in water comprising four successive larval instars followed by the mobile pupae. The adult stage occurs in open-air and constitutes the reproductive and dispersal phase. The mosquito characteristics regarding mating, physiology and behaviour of reproduction, fecundity and fertility are also described. Then elements are given on Ae. aegypti life table analysis, interspecific breeding, and the effect of the bacteria Wolbachia on the mosquito reproduction.
Life cycle
Four life stages
The life cycle of all species of mosquitoes, including Ae. aegypti, corresponds to the holometabolous type (Gordh, 2001) which is basically characterised by complete metamorphosis and four distinct life stages: egg; larva; pupa; and adult (Figure 2.1). The development cycle depends directly on the presence of water and ambient temperature. In warm days with temperatures averaging 25°C, development of eggs into adults is completed in a little more than 1 week. In the case of cool days, development may occur over a period of months (Foster and Walker, 2002). The stages are described below.
Breeding sites
The females lay individual eggs above the water level within fresh water held in natural breeding sites including holes in trees, bamboo trunks, hollow rocks, plant axilla, coconut shells, and leaves. Females will also oviposite on the inner wall of various artificial containers such as tanks, vases, jars, tires, drums, buckets, pots, cans, scrap metal and gutters (Nelson, 1986; Ulloa et al., 2010; Pilger et al., 2011), distributed inside houses or in their yards (Kampen and Schaffner, 2008).
The variability in the preference of the different types of containers as sites for oviposition by female Ae. aegypti depends on the availability of artificial containers, the degree of urbanisation and the season (Mogi and Mokry, 1980; García-Rejón et al., 2011; Rubio, Cardo and Vezzani, 2011).
Egg stage and embryonic development
Eggs can survive dry conditions for months and hatch once submerged in water, thus enhancing dissemination during the rainy periods. This survival ability of Ae. aegypti populations to dry seasons, combined with their intensive spread during rainy seasons, makes the control of Ae. aegypti very difficult (Nelson, 1986; Service, 2012).
There has been some research on the correlated effects of temperature and humidity on the eggs of Ae. aegypti. Experimental studies indicate that 20% of eggs remain viable after 6 months in 98% humidity (Luz et al., 2008). In Japan, Sota and Mogi (1992) measured survival times of eggs from several Aedes species including Ae. aegypti and Ae. albopictus under 3 different humidity conditions (42%, 68% and 88% of relative humidity) at 25°C, showing that Ae. aegypti survived longer than Ae. albopictus at all humidity conditions. Sota and Mogi (1992) attributed this to egg volume, with Ae. aegypti having the greatest egg volume and thus the greatest ability to resist desiccation. Juliano et al. (2002) also found the effects of temperature and humidity on egg mortality significantly different between the two species, with Ae. albopictus experiencing much higher mortality at all combinations except at the highest humidity. The maximum temperature limit for embryogenesis is 35°C and the minimum 12°C and below; and for egg viability optimal temperature ranges between 16-31°C, and with relative humidity above 80% (Farnesi et al., 2009). In a recent study, Thomas et al. (2012) found that eggs of a tropical strain of Ae. aegypti could survive at a threshold of 2°C for 24 hours only before hatching ceased. Egg survival at temperatures below freezing is therefore extremely unlikely.
The first 48 hours of embryonic development are critical and microclimatic factors are crucial for embryo survival (Thirión, 2003; Farnesi et al., 2009). The eggs of aedine mosquitoes usually enter a diapause-like state (suspension of development or quiescence) in unfavourable weather conditions (such as low temperature and humidity). They will hatch asynchronously several weeks or even months after being deposited with the return of more favourable conditions (Gillett, Roman and Phillips, 1977; Jeffery et al., 2012). In a natural setting, flooding from rainfall induces a physicochemical stimulus that results in egg hatching. Similarly, eggs are stimulated to hatch when submerged as the water level rises in water storage containers which are in everyday use (Koenraadt and Harrington, 2008). Additionally, other types of stimuli have been associated with hatching, for example, the low concentration of oxygen dissolved in water (Judson, 1960) and the presence of some water-soluble compounds or organisms in the water as a result of microbial activity (Gillett, Roman and Phillips, 1977; Ponnusamy et al., 2011).
Larval and pupal stages
The larval and pupal stages are strictly aquatic. Larval development begins with the first of four instars, each larger than the last. Passing from one larval stage to the next is accomplished by the moulting of chitinous skin that is shed, allowing growth and development of the next instar. Complete larval development typically lasts five to seven days and ends when the fourth instar larva develops and reaches the pupal form (Thirión, 2003). Larvae are omnivorous and spend most of their time feeding with the help of oral silks arranged in a fan which is used to filter particles of suspended organic matter and microorganisms in the water. They also graze organic matter on the bottom and sides of the flooded container (Colvard, 1978). The larvae feed in the water on protozoa, bacteria, yeasts and algae, both at the bottom of the habitat as well as in the water column (Ponce, 1999).
The duration of the aquatic phase of Ae. aegypti from first instar larvae to adult emergence, in the laboratory with water temperature at 24-27°C and no interspecific competition, is 8.42 days on average, with a range of 7.9-9.0 days. However, for both Ae. aegypti (Hancock et al., 2016) and Ae. albopictus (Sánchez-Hernández, 2011), the development time of larvae is significantly increased by competition for the limited amount of food in containers where the time to pupation can extend up to eight weeks.
Larval development is also favoured by the high prevalence of bacteria such as Aeromonas hydrophila/caviae, Klebsiella oxytoca, Pseudomonas sp., and Enterobacter cloacae in artificial breeding sites (tires, tanks, others) (Ulloa, 1996). These bacteria are potential food sources for larvae of Ae. aegypti. This study also revealed that discarded tires were the most important in terms of persistence in mosquito density and production of larvae. In this regard, Manrique-Saide et al. (1998) reported that the average time for immature stages of Ae. aegypti to develop in used tires was 11.15 to 12.95 days. Temperature, diet, density and their two-way interactions are all significant factors in explaining development rate variation of the larval stages of Ae. aegypti mosquitoes (Couret and Benedict, 2014).
The pupa is the last aquatic developmental stage, usually lasting between 2.0 and 3.6 days under optimal conditions (Focks et al., 1981; Nelson, 1986; Manrique-Saide et al., 1998). This stage is mobile (although non-feeding), and swims actively within the container in response to external stimuli such as vibrations and changes in light intensity.
Arrivillaga and Barrera (2004) determined the duration of the whole aquatic development phase (from first larval instar to adult) of Ae. aegypti in the laboratory associated with different levels of starvation for the immature stages. Development times varied between 8.5 days and 18.5 days with faster growth associated with increased food, highest water levels, and reduced density of larvae. Moreover, a comparative study between an Ae. aegypti wild type strain and a genetically engineered (GE) line1 showed a shorter time of pupation for the GE line (one day on average) as compared to the wild type strain, with this difference being more pronounced for females (1.4 days) than for males (0.9 day) (Bargielowski et al., 2011).
Adult stage
The adult or imago of the genus Aedes, like other groups of mosquitoes, is the reproductive and dispersal stage. Emergence of adult Ae. aegypti is usually crepuscular with adults released from the pupal exuviae performing an initial flight to a dry, resting place. The initial 24-hour period post-emergence is the teneral period, a physiological state during which the exoskeleton hardens and sexual maturation occurs (Clements, 2000). The teneral phase results in a fully mature aerial adult capable of flight and mating. Males are the first to emerge and a balanced sex ratio is produced, although sex ratios can be skewed by the presence of other competing species (Sánchez-Hernández, 2011).
The adult life expectancy varies from 10-35 days for female mosquitoes (Goindin et al., 2015) and 3-6 days for male mosquitoes (Clements, 2000) although this is highly dependent on temperature, being shorter in tropical regions and longer in more temperate climates, etc.
The dispersal range of adults is variable and is influenced by a variety of factors including the sex of the mosquito, density of human hosts, availability of breeding sites, abundance of plants in houses, as well as composition and configuration of ecological landscape (Reiter et al., 1995; Martinez-Ibarra et al., 1997; Rubio, Cardo and Vezzani, 2011). More information is given under Dispersal sub-section in Chapter 4.
Reproduction
Mating
Mosquitoes utilise sexual reproduction to produce new generations. Within 2-3 days after emergence, both sexes mate, and females can take a blood meal which is required for egg development (Lehane, 1991). These two activities often occur simultaneously because males are attracted to both the vertebrate host and the females, thus facilitating mating (Nelson, 1986).
The sound emitted by the flight frequency of females is used by males to locate and copulate with them (Brogdon, 1994). A source of attraction of a male to a female is the sound made by the beating of her wings during flight (Cator et al., 2009; Cator and Harrington, 2011). However, mating after engorgement of the females is rare because once the female has taken a blood meal, she must beat her wings more rapidly to carry her increased weight and the wing-beat frequency is no longer attractive to the male (Nelson, 1986; Cator et al., 2009).
During mating, the male clasps the tip of the female abdomen with his terminalia and inserts his aedeagus into the genital chamber. The female bursa copulatrix becomes filled with male sperm that passes within two minutes to the spermatheacae where they are stored prior to fertilisation of the eggs (Nelson, 1986).
Ae. aegypti females generally mate only once, since a single insemination event allows sufficient sperm to be stored within the spermatheacae to fertilise all the eggs that a female will develop during her lifetime. In addition, the seminal fluid proteins transferred from the male during mating render females unreceptive and refractory to further copulation (Sirot et al., 2008; Avila et al., 2011; Helinski et al., 2012). Thus, once mated, Ae. aegypti females are generally not responsive to additional matings for the duration of one or more egg-laying cycles (Cator et al., 2009). They may remate, however, if the spermatheacae is not adequately filled. Results from laboratory studies have revealed that 14% of females are involved in multiple matings (polyandry) within a 48-hour period (Helinski et al., 2012). Polyandry in a natural population of Ae. aegypti is low (6.25%), but also likely an underestimate and is within the range of polyandry estimates in other mosquito species (Richardson et al., 2015).
Laboratory studies have determined that the body size of male Ae. aegypti is a major predictor of total spermatozoa number, with significantly greater sperm numbers in larger males (2.27 mm wing length) versus smaller males (1.85 mm wing length) within the same age group (Ponlawat and Harrington, 2007). Other studies have shown that under field conditions, larger males inseminated females with more sperm than smaller ones and that older males transferred the greatest number of sperm to females (1 152 sperm by 1-day-old males compared to 1 892 sperm by 10-day-old males). At the same time, larger females successfully mated with males more often than smaller females, especially with older males (> 25-day-old) (Ponlawat and Harrington, 2009).
Physiology of reproduction
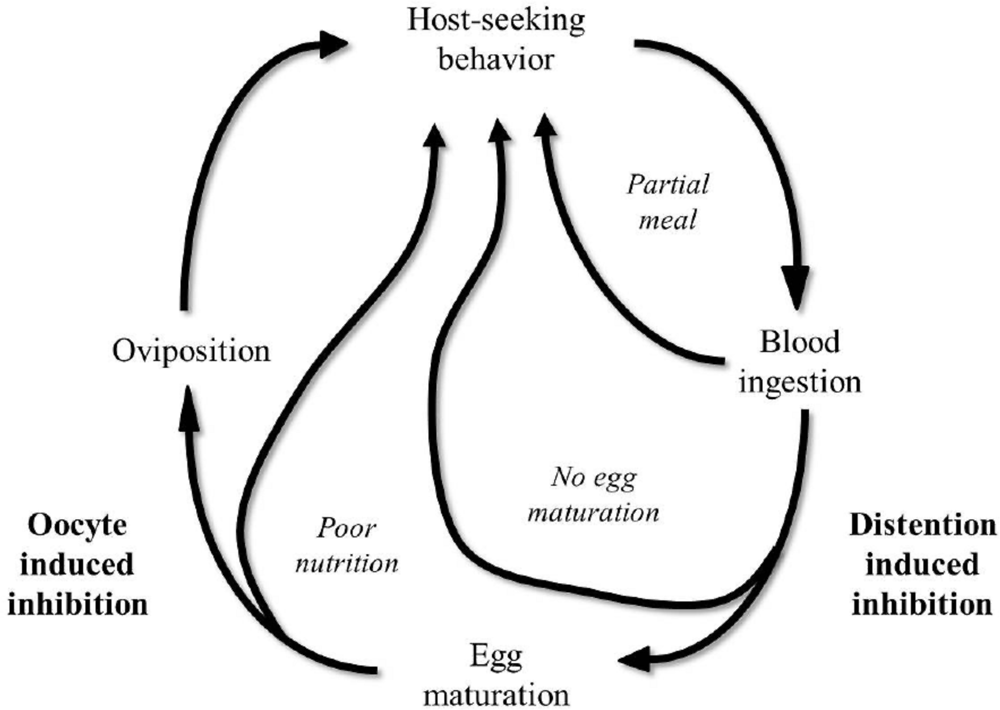
From the biological point of view, the physiological condition and the time required for females to carry out the digestion of a blood meal, maturation of the follicles, and subsequent oviposition constitutes a strategy of reproductive competition; a strategy for competition between females for resources required for reproduction (Wheeler, 1996). The gonotrophic cycle includes the search for the host, the ingestion of a blood meal, the digestion of the blood, the maturation of ovaries. It is completed with the laying of eggs once females have found an appropriate oviposition site (Beklemishev, 1940). Figure 2.2 graphically describes the integration of the physiological processes (summarised as host-seeking, ingestion and digestion of blood, egg maturation and oviposition) associated with feeding and reproduction of Ae. aegypti as suggested by Klowden (1994).
The host-seeking behaviour of Ae. aegypti is closely associated with anthropogenic environments, in and around homes and other places that people frequent. During host-seeking behaviour in mosquitoes, visual, thermal and olfactory stimuli all contribute to host location, but olfaction is probably the dominant sensory mode used for this purpose (Bowen, 1996).
The visual capacity of Aedes mosquitoes to distinguish between various optical stimuli such as luminous reflectance, vertical contrast, and movement (Muir, Kay and Thorne, 1992; Hoel, Kline and Allan, 2009), as well as their preference of resting on black, stationary objects and non-reflective surfaces such as clothing, are characteristics that have served in the development of various entomological sampling devices and traps, e.g. the BG Sentinel trap, ovitraps, gravid Aedes Traps and BDV tent trap (Fay and Prince, 1970; Muir, Kay and Thorne, 1992; Edman et al., 1997; Kroeckel et al., 2006; Silver, 2007; Casas Martínez et al., 2013; Eiras, Buhagiar and Ritchie, 2014).
With regard to the role of olfaction in host-seeking behaviour, carbon dioxide (CO2) is involved in both short-range and long-range attraction. Olfactory cues that are primarily involved in long-range attraction include skin emanations, exhaled air and urine. Each of these is attractive to all mosquito species. Attraction is caused by a mixture of several host emanated compounds (Takken, 1991). Lactic acid in the presence of CO2 is attractive, and lactic acid-sensitive neurosensilla are present on the antennae of Ae. aegypti. Other host-produced chemicals are also attractive. The plant-derived odorant linalool oxide, in combination with CO2 is also an effective long-range attractant (Nyasembe et al., 2015).
The amount of blood ingested by a female Ae. aegypti mosquito (> 2.5 µl on average) can affect its host-seeking response. The suspension of host-seeking behaviour is caused by abdominal distension due to the ingested blood, or due to hormonal inhibition (Klowden and Lea, 1978).
To meet the adult female’s energy and reproductive needs this species has adopted a strategy that includes reduced consumption of plant carbohydrates, highly focused blood feeding on humans, and frequently engaging in multiple blood feedings (Scott and Takken, 2012). Ae. aegypti almost exclusively fed on humans (99%) as a single host species, and 97% of multiple-host blood meals included at least one human host. A low frequency of other hosts, including bovine, swine, cat, rat and chicken were detected, but they represented less than 1% of blood meals (Ponlawat and Harrington, 2005). Both males and females can feed on plant juices (nectar), damaged fruits, damaged and intact vegetative tissue, and homopterans (aphids) which act as an energy source for their physiological maintenance and locomotion (Clements, 2000). Carbohydrate consumption rates (fructose) ranged from 1% to 27% for females and 9% to 65% for males (males are not hematophagous) (Van Handel et al., 1994; Martínez-Ibarra et al., 1997). Sugar feeding in Ae. aegypti is believed to be facultative because studies indicate that in the absence of human hosts, females showed higher fructose feeding rates, up to 74% (Van Handel et al., 1994).
The usual gonotrophic cycle of Ae. aegypti is described above. However, lack of association between blood feeding and ovogenesis, a term known as gonotrophic discordance, is fairly common in Ae. aegypti. This concept is defined as the need for multiple blood meals during a single gonotrophic cycle. The occurrence of multiple partial meals for a gonotrophic cycle (Feinson and Spielman, 1980; Clements, 1992) and reduced feeding success may be due to host defensive behaviour, body size of females and local female Ae. aegypti mosquito abundance (Klowden and Lea, 1978; Clements, 1992). The habit of feeding on blood twice during one gonotrophic cycle depends greatly on the size and hence stored energy reserves of the teneral female (Takken et al., 1998).
Some field studies with Ae. aegypti females demonstrated that 88% of all detectable meals were identified as being from a single host (human) and only 7% of all the females had taken multiple meals (Scott et al., 1993). Engorged females in Thailand revealed that half to one-third imbibed two or more blood meals in a 36-hour time period. On average, the human biting rate was high, with 0.63-0.76 blood meals per day (Scott et al., 2000). Multiple blood meals were also recorded using histological examination.
Protein obtained from the blood meal supplies the amino acids needed for vitellogenin synthesis, which is a protein critical for egg production in the female Ae. aegypti mosquito. In general, the post-ingestion digestion of blood takes about 38-48 hours in the midgut (MG) of Ae. aegypti (O'Gower, 1955; Gaio et al., 2011) and is dependent upon temperature and, to a lesser extent, humidity (Shlenova, 1938; West and Eligh, 1952).
Many bacteria live and multiply in the MG of Ae. aegypti, contributing to digestion, nutrition, and development of their host. The reduction in these symbiotic MG bacteria (primarily Enterobacter sp. and Serratia sp.) can affect the lysis of red blood cells, subsequently retarding protein digestion, depriving the mosquito of essential nutrients and eventually affecting oocyte maturation resulting in the production of fewer viable eggs (Gaio et al., 2011).
The gonotrophic cycle duration is operationally defined as the average number of days that gravid mosquitoes took to oviposit after taking a blood meal. From a human health perspective, the gonotrophic cycle is one of the most important physiological processes in the life of mosquitoes vectoring dengue and represents an essential epidemiological component in the model of vectorial capacity. It is a significant and determining biological aspect in the population dynamics of Ae. aegypti and Ae. albopictus, both of which can coexist in urban, suburban, and rural regions with endemic dengue and other arboviral diseases. Bacon (1916) in West Africa found that the first meal was taken one to two days after emergence and subsequent meals taken after each oviposition at about three-day intervals. The development of follicles from stage I to V (Christophers, 1911), takes 1.67 days during the first gonotrophic cycle of Ae. aegypti females when fed with a blood supply to repletion, and maintained at an average temperature of 28.9°C. The maturation of eggs can extend up to 2.7 days with an average temperature of 26.2°C (Tamayo-Domínguez, 2011). Additionally, female Ae. aegypti took 2.8 days to complete the first gonotrophic cycle when the average temperature was 26.2°C (Tamayo-Domínguez, 2011).
Because the processes of feeding and reproduction are closely related in most anautogenous (requiring a blood meal) anthropophilic mosquitoes like Ae. aegypti, therefore larval nutritional regimen, body size of newly-emerged adults, and the quantity and quality of blood ingested by females are key considerations (Macdonald, 1956). In mosquitoes, egg production is a cyclic process; therefore, with each successive reproductive or gonotrophic cycle a batch of oocytes matures and a new set of follicles forms within the germaria, separates and starts development. In Ae. aegypti, secondary follicles appear when the primary follicles enter the previtellogenic resting stage (Clements, 2000).
Once ovogenesis, which is asynchronous (Clements, 1992), is complete (or reaches Christophers’ stage V), the priority of a female Ae. aegypti is to search for an oviposition site. Typically, eggs are deposited in naturally occurring collections of fresh water (such as coconut shells, leaves and axils of plants, tree holes, hollows of rocks) and various artificial containers made of plastic, glass, ceramic or metal, while holding temporal (e.g. tires, vases, bottles, kitchenware, scrap metal) and/or permanent water sources (pools, drums, tanks, etc.) that provide both habitat and food for immature life stages (Thavara et al., 2001; Vezzani and Schweigmann, 2002; García-Rejón et al., 2011). Oviposition sites may be located inside and outside human habitations, as well as in non-residential places such as cemeteries, workshops, junkyards, tire repair facilities and vacant plots. There have been reports of Ae. aegypti larvae being found in the surface clear water layer of septic tanks (Burke et al., 2010), but this is not frequent and usually occurs where the lid is cracked or broken, providing the female access; nonetheless, septic tanks can be prolific producers (Barrera et al., 2008). Breeding sites also can include those that might contain brackish water such as boats or man-made containers at coastal edges or on beaches (Ramasamy et al., 2011). Waste material containers that are situated in areas with overhanging vegetation provide more favourable habitats as the breeding site is both shaded from intense sunshine and the build-up of heat and provides a ready source of detritus and bacteria for larval consumption. These containers are usually breeding sites for mosquitoes only during the rainy season in countries with wet and dry seasons, but the eggs are resistant to desiccation and can remain in suitable containers until rains of the following season. These desiccated eggs form what is known as the egg bank.
The choice of an egg-laying site by Ae. aegypti is influenced by the presence of conspecific larvae and pupae, the container fill method, container size, lid and sun exposure (Wong et al., 2011). Surprisingly, egg-laying females were most attracted to sites containing other immature Ae. aegypti, rather than to sites containing the most food. Physical attributes of oviposition sites, such as size, light-dark contrasts and specular reflectance from water surfaces, also play a significant role in oviposition site selection (Harrington et al., 2008). Characteristics of oviposition sites can vary according to the geographic and sociocultural context such as region, country and location. The degree of landscape modification (urban-rural) is also a factor (Kittayapong and Strickman, 1993; Honório et al., 2009), as well as intra- and interspecific competition (Chadee, Corbet and Greenwood, 1990; Braks et al., 2004; Sánchez-Hernández, 2011).
Behaviour of reproduction
Ae. aegypti is recognised as a highly anthropophilic, endophilic, endophagic, and day-biting species (Scott and Takken, 2012; Brown et al., 2014; McBride et al., 2014). These designations are based on activity patterns exhibited by this mosquito around the world. An important aspect of the bionomics of Ae. aegypti that contributes to its efficiency as an epidemiological vector is the close association with domestic habitats (Scott et al., 2000). Adult mosquitoes frequently reside indoors in human dwellings, most commonly in bedrooms (60.3% to 63.5%) followed by living/dining rooms (9.3% to 18.4%), kitchens (7.5% to 9.7%) and bathrooms (6.6% to 11.5%) (García-Rejón et al., 2008; Casas-Martínez, 2013). Immature forms develop primarily in artificial containers such as cans, jars, tires and buckets (Winch et al., 1992; García-Rejón et al., 2011). In Chennai, one of the major metropolitan areas in India, intradomiciliary cement tubs containing water for multi-purpose works were mostly preferred by the Ae. aegypti immature forms for development (Arunachalam et al., 2010). More details are given under Chapter 4.
Mosquitoes are exposed to daily changes in environmental light-dark cycles along with variations in humidity and temperature. Adaptation to these changes is seen in the form of specific behaviours, which are in turn linked to the expression of specific endogenously-controlled genes. Ae. aegypti is a major vector of arbovirus in many countries,2 therefore the ethological study of this mosquito is crucial to better understand their behaviour, the dynamics of transmission of the viruses, as well as to optimise the entomological surveillance and increase the efficiency of vector control (Lima-Camara, 2010; Sivagnaname and Gunasekaran, 2012).
There are two significant copulation peaks in indoor housing, an early-morning peak between 6h00 and 8h00 (25% of events) and a pre-sunset peak from 16h00 to 18h00 (24% of events). The outdoors copulation periodicity presents almost the same pattern in the timing, with 30% of events during the early morning peak and 25% of events during the pre-sunset peak. Observations in insectary have shown similar copulation patterns. Studies indicated that 38.6% of copulating females collected in and around breeding sites were nulliparous and not inseminated, whereas over 85% of the copulating females found indoors were parous, suggesting that successful insemination encounters occur at alternative sites (such as around the human host). Furthermore, males may not be able to detect the difference between virgin and mature, parous female mosquitoes (Chadee and Gilles, 2013).
Oviposition is also diurnal and bimodal, both indoors and outdoors, with consistent peaks at 6h00-8h00 and 16h00-18h00 (Chadee and Corbet, 1989, 1990; Corbet and Chadee, 1989). The oviposition activity intensifies during the rainy season due to increased availability of water filled containers and mosquito population abundance.
Visual observations of the mating behaviour of Ae. aegypti have shown that males swarm around the feet and lower legs of a sitting/standing human host, flying in a horizontal figure of eight pattern. Mating was usually initiated in flight at a height of not more than one metre from the ground. Copulating pairs have been observed in flight, on human bodies, on their trousers and on the ground (Hartberg, 1971). Mating also occurs near adult oviposition sites and resting sites. Tests carried out by Jong and Knols (1996), demonstrated that Ae. aegypti prefers to bite the head and upper part of the trunk of persons lying in prone or supine position but will often bite on the lower legs beneath tables and when the host is seated.
The host-seeking behaviour and biting activity of Ae. aegypti are closely related, therefore, both events describe overlapping biorhythms. Many authors have documented that males and females show a bimodal flying and landing activity and that the periodicity is the same for nulliparous, parous, inseminated or uninseminated females, all activity being predominantly diurnal, with sharp peaks at post-sunrise and pre-sunset (as reported above) in intra-, peri- and extradomiciliary sites (Trpis et al., 1973; Corbet and Smith, 1974; Casas-Martínez et al., 2013). Landing activity patterns of Ae. aegypti are influenced by environmental factors (for example electrical lighting in and around houses), both indoor and outdoor, and in urban and rural areas (Chadee and Martinez, 2000).
Fecundity and fertility
Some mosquito strains or species are able to lay eggs without taking a blood meal, a trait named autogeny. This may allow populations to persist through times or places where vertebrate hosts are scarce. Environmental and genetic factors determine whether the mosquito Ae. aegypti lays eggs without a blood meal (Ariani et al., 2015). Autogeny is increased by growth at a temperature of 28°C (compared with 22°C), good nutrition of larval stages and feeding on higher concentrations of sugar solution during the adult stage. There appears to be a genetic component to autogeny which allows adult females from some strains to utilise amino acids from fat stores from the larva stage instead of obtaining these nutrients from a blood meal. Genetic differences associated with autogeny also affect fecundity in autogenous Ae. aegypti strains as shown by blood feeding behaviour (Christophers, 1960), quantity and quality of blood acquired (Klowden and Lea, 1978; Clements, 1992) and insemination status (Lavoipierre, 1958).
Mosquito body size has been linked to longevity, the number of eggs per batch and vector competence, and it is therefore an important measure of mosquito fitness (Siegel et al., 1992). The average body size corresponding to a wing length of 2.6 mm was associated with 61.23 ± 29.15 eggs per batch (Tamayo-Domínguez, 2011).
Life table analysis, under natural (and laboratory) conditions
A life table of aquatic phase Ae. aegypti grown under favourable laboratory conditions (temperature maintained at 28 ± 1°C, humidity 70 ± 10%, app. 50 larvae in a 6-inch x 8-inch tray, yeast + dog biscuit powder, or an alternative, as food on alternate days) suggests that natural mortality during development of larval stages is initially low (1%, stage I) and then increases (9%, stage II; 34%, stage III; 34%, stage IV). Mortality then decreases during the pupal stage (6%). When grown in the laboratory, approximately 48% of eggs survive to adults (Sánchez-Hernández, 2011).
Observed patterns of coexistence/exclusion of Ae. albopictus and Ae. aegypti in the field (Murrell and Steven, 2008) may be due to variation in detritus type. Experimental trials confirm competitive asymmetry in favour of Ae. albopictus with oak, pine, rubber (Tyagi et al., 2006) or insect detritus. Certain detritus types may eliminate interspecific competition among the larvae of these species (Murrel and Steven, 2008), thereby allowing for stable coexistence. Desiccation and thermal tolerance of eggs are also factors affecting co-existence (Juliano et al., 2002). More details on the biotic interactions in the landscape are given in the related section of Chapter 4.
In general, the effects of microclimatic factors (temperature, humidity and rainfall) and other environmental variables (food source, breeding sites and shelters) in the life cycle of the mosquito Ae. aegypti and generation time have been well documented, in either natural or controlled conditions in an insectarium or laboratory. Environmental changes affect all life stages of the mosquito, influence their survival and thus their ability to transmit pathogens. Low humidity, for instance, can negatively affect adult survival and may decrease the vector population. Frequency and host type of blood meal influence fecundity and female survival (Christophers, 1960; Nelson, 1986; Rueda et al., 1990; Day, Edman and Scott, 1994; Carrington et al., 2013).
Interspecific breeding
Harper and Paulson (1994) examined the dynamics of interspecific and intraspecific mating between Florida strains of Ae. aegypti and Ae. albopictus. In non-choice experiments where conspecific males were not available, dissection of the spermatheacae showed that interspecific insemination was an infrequent event. Few eggs were produced from interspecific crosses and all were non-viable. The frequency of interspecific mating was not increased when the hind tarsi of females were removed, eliminating a significant mechanism for fending off unwanted courtship. When held with males of both species, females mated with conspecifics and oviposited without regard to the presence of other species. In low-density experiments in which a single female of either species is caged with an excess of males of the other species, the conspecific male always located and inseminated the female. However, the presence of females of the other species has some negative influence on the intraspecific mating success in male Ae. aegypti, most likely due to misdirected courting or mating efforts (Bargielowski, Blosser and Lounibos, 2015).
Additional studies further suggest that matings of Ae. aegypti with Ae. albopictus do not produce viable offspring in the laboratory (Harper and Paulson, 1994; Nazni et al., 2009). Forced matings in the laboratory between wild-type Ae. aegypti and Ae. albopictus yielded eggs but they were not viable, and when bleached were shown to have no embryos (Nazni et al., 2009). More recently a study showed that there is cross-species insemination in the field between Ae. aegypti and Ae. albopictus (Tripet et al., 2011), but these interspecific matings encounter many barriers and occur at low frequencies (a single Ae. albopictus was found to have Ae. aegypti sperm in this study, and three Ae. aegypti females were inseminated by Ae. albopictus), resulting in no viable progeny.
These results indicate that significant reproductive isolation exists between Ae. aegypti and Ae. albopictus. This occurs at both the prezygotic level (very low mating frequency) and the postzygotic level (non-viable progeny).
In rare cases, viable hybrids resulting from cross-mating between Ae. aegypti females and Ae. albopictus males have occurred in laboratories (Martínez-López et al., 2014). Eggs obtained from this cross-mating were viable, and the larvae and pupae showed development in seven days. Therefore, it is possible that viable hybrids can be produced experimentally, but this is rare and may be restricted to matings of only particular strains of each species. As reported in the previous section, there are important reproductive barriers existing between these two species living in sympatry in natural environments (Harper and Paulson, 1994; Nazni et al., 2009) and the possibility of hybridisation is unlikely, given the probable sterility of F1 hybrids (Haldane’s rule).
Effect of Wolbachia on reproduction
Wolbachia pipientis is a monophyletic group of maternally inherited, gram-negative, endosymbiotic bacteria, related to the Ehrlichia, Anaplasma and Neorickettsia genera, all being members of Alphaproteobacteria (O’Neill et al., 1992; Lo et al., 2007). In recent years, evidence has been accumulated that shows Wolbachia infections affect several aspects of host biology, physiology, immunity, ecology, evolution and reproduction (Bourtzis, Braig and Karr, 2003; Bourtzis and Robinson, 2006; Werren, Baldo and Clark, 2008; Saridaki and Bourtzis, 2010). This bacterial group is widespread and abundant among insect species and has been associated with the induction of a number of reproductive outcomes including the death of males (Hurst et al., 2000), feminisation (Rousset et al., 1992), parthenogenesis (Stouthamer, Breeuwer and Hurst, 1999) and, most commonly, cytoplasmic incompatibility (Yen and Barr, 1973; O’Neill et al., 1997; Nirgianaki et al., 2003).
The cytoplasmic incompatibility (CI) results in the generation of unviable offspring when an uninfected female mates with a Wolbachia-infected male (McGraw et al., 2001). In contrast, Wolbachia-infected females can produce viable progeny when they mate with both infected and uninfected males, resulting in a selective reproductive advantage over uninfected females (Hoffmann and Turelli, 1997). This CI phenotype is induced by Wolbachia in mosquito species and allows the maternally-transmitted Wolbachia to efficiently invade host populations without being infectious or moving horizontally between individuals (Hoffmann and Turelli, 1997).
The ability of Wolbachia to manipulate diverse functional systems of its hosts (Bourtzis et al., 2014), particularly reproduction, has led to the proposal and the development of promising symbiont-based strategies aimed at the control of insect pests and disease vectors including mosquito species. Different Wolbachia species/strains can be naturally found in Aedes mosquitoes, for example, Ae. albopictus, Ae. polynesiensis and Ae. scutellaris, but not in Ae. aegypti. Thus, the use of Wolbachia for Ae. aegypti control via CI has required its transinfection from naturally infected insect species (Ye et al., 2013; Joubert et al., 2016) and currently includes the wAlbB strain (from Ae. albopictus) and the wMelPop-CLA (cell-line-adapted) and wMel strains (from Drosophila melanogaster). More information on the use of Wolbachia as a biological control for virus transmission is given under Annex A.
References
Ariani, C.V. et al. (2015), “Environmental and genetic factors determine whether the mosquito Aedes aegypti lays eggs without a blood meal”, The American Journal of Tropical Medicine and Hygiene, Vol. 92, No. 4, pp. 715-721.
Arrivillaga, J. and R. Barrera (2004), “Food as a limiting factor for Aedes aegypti in water-storage containers”, Journal of Vector Ecology, Vol. 29, No. 1, pp. 11-20.
Arunachalam, N. et al. (2010), “Eco-bio-social determinants of dengue vector breeding: A multi-country study in urban and periurban Asia”, Bulletin of the World Health Organization, Vol. 88, No. 3, pp. 173–184.
Avila, F.W. et al. (2011), “Insect seminal fluid proteins: Identification and function”, Annual Review of Entomology. 2011, Vol. 56, pp. 21–40.
Bacon AW. (1916), Investigation Report of Yellow Fever Commission West Africa”, in Report of the entomological investigation undertaken for the Yellow Fever (West Africa) Commission for the year August 1914, to July 1915 (Quoted by Edwards, 1941).
Bargielowski, I., E. Blosser and L.P. Lounibos (2015), “The effects of interspecific courtship on the mating success of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) males”, Annals of the Entomological Society of America, Vol. 108, No. 4, pp. 513–518.
Bargielowski, I. et al. (2011), “Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti”, PLoS One, Vol. 6, No. 6: e20699.
Barrera, R. et al. (2008), “Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control”, Medical and Veterinary Entomology, Vol. 22, No. 1, pp. 62-69.
Beklemishev, W.N. (1940), “Gonotrophic rhythm as a basic principle of the biology of Anopheles”, Vopr Fiziol Ekol Malar Komara, Vol. 1, pp. 3-22.
Bourtzis, K., H.R. Braig and T.L. Karr (2003), “Cytoplasmic incompatibility”, in K. Bourtzis and T.A. Miller (eds.), Insect Symbiosis, Vol. 1, CRC Press, pp. 217-246.
Bourtzis, K. and A.S. Robinson (2006), “Insect pest control using Wolbachia and/or radiation”, in K. Bourtzis and T.A. Miller (eds.), Insect Symbiosis, Vol. 2, CRC Press, pp. 225-246.
Bourtzis, K. et al. (2014), “Harnessing mosquito-Wolbachia symbiosis for vector and disease control”, Acta Tropica, Vol. 132 (Suppl.), S150-S163.
Bowen, M.F. (1996), “Sensory aspects of host location in mosquitoes”, in CIBA Foundation Symposium 200, pp. 197-201 and 226-232.
Braks, M.A. et al. (2004), “Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil”, Annals of Entomological Society of America, Vol. 97, No. 1, pp. 130-139.
Brogdon, W.G. (1994), “Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae)”, Journal of Medical Entomology, Vol. 31(5), pp.700-703.
Brown, J.E. et al. (2014), “Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito”, Evolution, Vol. 68, No. 2, pp. 514-525.
Burke, R.L. et al. (2010), “Examination of a miniaturized funnel trap for Aedes aegypti (Diptera: Culicidae) larval sampling”, Journal of Medical Entomology, Vol. 47, No. 6, pp. 1231–1234.
Carrington, L.B. et al. (2013), “Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits”, PLoS ONE, Vol. 8, No. 3: e58824.
Casas-Martínez, M. (2013), “Bionomía comparativa de Aedes aegypti y Aedes albopictus y sus implicaciones en la transmisión del dengue en el sur de México” [Comparative bionomy of Aedes aegypti and Aedes albopictus and its implications for the transmission of dengue fever in southern Mexico], Doctoral thesis, Tapachula.
Casas-Martínez, M. et al. (2013), “A new tent trap for monitoring the daily activity of Aedes aegypti and Aedes albopictus”, Journal of Vector Ecology, Vol. 38, No. 2, pp.277-288.
Cator, L.J. and L.C. Harrington (2011), “Harmonic convergence and sexy sons: Indirect benefits associated with acoustic signals in the dengue vector”, Animal Behaviour, Vol. 82, No. 4, pp. 627-633.
Cator, L.J. et al. (2009), “Harmonic convergence in the love songs of the dengue vector mosquito”, Science, Vol. 323, No. 5917, pp. 1077-1079.
Chadee, D.D. and J.R.L. Gilles (2013), “The diel copulation periodicity of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) at indoor and outdoor sites in Trinidad, West Indies”, Acta Tropica, Vol. 132S, pp. 91-95.
Chadee, D.D. and P.S. Corbet (1990), “A night-time role of the oviposition site of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae)”, Annals of Tropical Medicine and Parasitology, Vol. 84, No. 5, pp. 429-433.
Chadee, D.D. and P.S. Corbet (1989), “Diel patterns of oviposition of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, W. I.: A preliminary study”, Annals of Tropical Medicine and Parasitology, Vol. 84, No. 1, pp. 79-84.
Chadee, D.D., P.S. Corbet and J.J.D. Greenwood (1990), “Egg-laying Yellow Fever Mosquitoes avoid sites containing eggs laid by themselves or by conspecifics”, Entomology Experimental Applied, Vol. 57, pp. 295-298.
Chadee, D.D. and R. Martinez (2000), “Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies”, Journal of Vector Ecology, Vol. 25, No. 2, pp. 158-163.
Christophers, S.R. (1960), Aedes aegypti (L.) The Yellow Fever Mosquito. Its Life History, Bionomics and Structure, Cambridge University Press, Cambridge.
Christophers, S.R. (1911), “The development of the egg follicle in Anophelines”, Paludism, Vol. 2, pp. 73-78.
Clements, A.N. (2000), The Biology of Mosquitoes, Volume I: “Development, Nutrition and Reproduction” Second Edition, CABI Publishing, Oxford.
Clements, A.N. (1992), The Biology of Mosquitoes, Volume I “Development, Nutrition and Reproduction”, Chapman and Hall, London.
Colvard, J. (1978), “El comportamiento alimentario de los mosquitos”, Investigación y Ciencia, Spanish edition of Scientific American, Vol. 23, pp. 86-93.
Corbet, P.S. and D.D. Chadee (1989), “Incidence and diel pattern of oviposition outdoors of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, W. I. in relation to solar aspect”, Annals of Tropical Medicine and Parasitology, Vol. 84, No. 1, pp. 63-78.
Corbet, P.S. and S.M. Smith (1974), “Diel periodicities of landing of nulliparous and parous Aedes aegypti (L.) at Dar es Salaam, Tanzania (Diptera: Culicidae)”, Bulletin of Entomological Research, Vol. 64, pp. 111-121.
Couret, J. and M.Q. Benedict (2014). “A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae)”, BioMed Central Ecology, Vol. 14, No. 3, pp 1-15.
Day, J.F., J.D. Edman and T.W. Scott (1994), “Reproductive fitness and survivorship of Aedes aegypti (Diptera: Culicidae) maintained on blood, with field observations from Thailand”, Journal of Medical Entomology, Vol. 31, No. 4, pp. 611-617.
Edman, J.D. et al. (1998), “Aedes aegypti (Diptera: Culicidae) movement influenced by availability of oviposition sites”, Journal of Medical Entomology, Vol. 35, pp. 578-583.
Eiras, A.E., T.S. Buhagiar and S.A. Ritchie (2014), “Development of the Gravid Aedes Trap for the capture of adult female container-exploiting mosquitoes (Diptera: Culicidae)”, Journal of Medical Entomology, Vol. 51, pp. 200-209.
Farnesi, L.C. et al. (2009), “Embryonic development of Aedes aegypti (Diptera: Culicidae): Influence of different constant temperatures”, Memórias do Instituto Oswaldo Cruz, Vol. 104, No. 1, pp. 124-126.
Fay, R.W. and W.H. Prince (1970), “A modified visual trap for Aedes aegypti”, Mosquito News, Vol. 28, pp. 1-7.
Feinson, F.M. and A. Spielman (1980), “Nutrient mediated juvenile hormone secretion in mosquitoes”, Journal of Insect Physiology, Vol. 26, pp. 113-117.
Focks, D.A. et al. (1981), “Observations on container-breeding mosquitoes in New Orleans, Louisiana with an estimate of the population density of Aedes aegypti (L)”, American Journal of Tropical Medicine and Hygiene, Vol. 30, pp. 1329-1335.
Foster, W.A. and E.D. Walker (2002), “Mosquitoes (Culicidae)”, in G. Mullen and L. Durden (eds.), Medical and Veterinary Entomology, Academic Press, San Diego, pp. 203-262.
Gaio, A. de O. et al. (2011), “Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.)”, Parasites and Vectors, Vol. 4, pp. 105.
García-Rejón, J.E. et al. (2011), “Productive container types for Aedes aegypti immatures in Mérida, México”, Journal of Medical Entomology, Vol. 48, No. 3, pp. 644-650.
Gillett, J.D., E.A. Roman and V. Phillips (1977), “Erratic hatching in Aedes eggs: A new interpretation”, Proceedings of the Royal Society of London. Series B, Biological Sciences, Vol. 196, pp. 223-232.
Goindin, D. et al. (2015), “Parity and longevity of Aedes aegypti according to temperatures in controlled conditions and consequences on dengue transmission risks”, PLoS ONE, Vol. 10, No. 8: e0135489.
Gordh, G. (2001), A Dictionary of Entomology, compiled by G. Gordh with assistance by D. Headrick, CABI Publishing, Wallingford (United Kingdom) and Cambridge (Massachusetts).
Hancock, P.A. et al. (2016), “Density dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia”, Journal of Applied Ecology, Vol. 53, No. 3, pp. 785-793.
Harper, J.P. and S. Paulson (1994), “Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus”, Journal of the American Mosquito Control Association, Vol. 10, pp. 88-92.
Harrington, L.C. et al. (2008), “Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand”, Vector-Borne and Zoonotic Diseases, Vol. 8, No. 3, pp. 415–423.
Hartberg, W.K. (1971), Observation on the Mating Behavior of Aedes aegypti in Nature, World Health Organization, pp. 847-850.
Helinski, M.E. et al. (2012), “Evidence of polyandry for Aedes aegypti in semifield enclosures”, American Journal of Tropical Medicine and Hygiene, Vol. 86, No. 4, pp. 635-641.
Hoel, D., D. Kline and S. Allan (2009), “Evaluation of six mosquito traps for collection of Aedes albopictus (Skuse) and associated mosquito species in a suburban setting in north central Florida”, Journal of the American Mosquito Control Association, Vol. 25, pp. 47-57.
Hoffman, A.A. and M. Turelli (1997), “Cytoplasmic incompatibility in insects”, in S.L. O'Neill, A.A. Hoffman and J.H. Werren (eds), Influential Passengers, Oxford University Press, New York, pp. 42–80.
Honório, N.A. et al. (2009), “The spatial distribution of Aedes aegypti and Aedes albopictus in a transition zone, Rio de Janeiro, Brazil”, Cad Saude Publica, Vol. 25, No. 6, pp. 1203-1214.
Hurst, G.D. et al. (2000), “Male-killing Wolbachia in Drosophila: A temperature-sensitive trait with a threshold bacterial density”, Genetics, Vol. 156, No. 2, pp. 699-709.
Jeffery, J.A.L. et al. (2012), “Water level flux in household containers in Vietnam - A key determinant of Aedes aegypti population dynamics”, PLoS ONE, Vol. 7, No. 7: e39067.
Jong, R. and B.G. Knols (1996), “Selection of biting sites by mosquitoes”, in G.R. Bock and G. Cardew, Olfaction in Mosquito-Host Interactions, Ciba Foundation, England, pp. 1-331.
Joubert, D.A. et al. (2016), “Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management”, PLoS Pathos, Vol. 12, No. 2: e1005434.
Judson, C.L. (1960), “The physiology of hatching of aedine mosquito eggs: Hatching stimulus”, Annals of the Entomological Society of America, Vol. 53, pp. 688-691.
Juliano, S.A. et al. (2002), “Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes”, Oecologia, Vol. 130, pp. 458-469.
Kampen, H. and F. Schaffner (2008), “11. Mosquitoes”, in X. Bonnefoy, H. Kampen and K. Sweeney (eds.), Public Health Significance of Urban Pests, World Health Organization, WHO Regional Office for Europe, Denmark, pp. 347-386.
Kittayapong, P. and D. Strickman (1993), “Distribution of container-inhabiting Aedes larvae (Diptera: Culicidae) at a dengue focus in Thailand”, Journal of Medical Entomology, Vol. 30, No. 3, pp. 601-606.
Klowden, M.J. (1994), “Endogenous regulation of the attraction of Aedes aegypti mosquitoes”, Journal of the American Mosquito Control Association, Vol. 10, pp. 326-332.
Klowden, M.J. and A.O. Lea (1978), “Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.)”, American Journal of Tropical Medicine and Hygiene, Vol. 27, pp. 827-831.
Koenraadt, C.J.M. and L.C. Harrington (2008), “Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae)”, Journal of Medical Entomology, Vol. 45, No. 1, pp. 28-35.
Kroeckel, U. et al. (2006), “New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment”, Journal of the American Mosquito Control Association, Vol. 22, No. 2, pp. 229-238.
Lavoipierre, M.M. (1958), “Biting behavior of mated and unmated females of an African strain of Aedes aegypti”, Nature, Vol. 181, pp. 1781-1782.
Lehane, M.J. (1991), Biology of Blood-Sucking Insects. First Edition, Chapman and Hall, London, pp. 288.
Lima-Camara, T.N. (2010), “Activity patterns of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) under natural and artificial conditions”, Oecologia Australis Vol. 14, pp. 737-744.
Lo, N. et al. (2007), “Taxonomic status of the intracellular bacterium Wolbachia pipientis”, International Journal of Systematics and Evolutionary Microbiology, Vol. 57, pp. 654-657.
Luz, C. et al. (2008), “Impact of moisture on survival of Aedes aegypti eggs and ovicidal activity of Metarhizium anisopliae under laboratory conditions”, Memórias do Instituto Oswaldo Cruz, Vol.103, No.2, pp. 214-215.
Macdonald, W.W. (1956), “Aedes aegypti in Malaya: II larval and adult biology”, Annals of Tropical Medicine and Parasitology, Vol. 50, pp. 399-414.
Manrique-Saide, P. et al. (1998), “Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres”, Medical and Veterinary Entomology., Vol. 12, No. 4, pp. 386-390.
Martínez-Ibarra, J.A. et al. (1997), “Influence of plant abundance on nectar feeding by Aedes aegypti (Diptera: Culicidae) in southern Mexico”, Journal of Medical Entomology, Vol. 34, No. 6, pp. 589-593.
Martínez-López, Y. et al. (2014), “Cruzamiento interespecífico entre Aedes aegypti y Aedes albopictus en el laboratorio” [Interspecific breeding between Aedes aegypti and Aedes albopictus in laboratory], Revista Cubana de Medicina Tropical [Cuban Journal of Tropical Medicine], Vol. 66, No. 1, pp. 148-151.
McBride, C.S. et al. (2014), “Evolution of mosquito preference for humans linked to an odorant receptor”, Nature, Vol. 515, pp. 222–227.
McGraw, E.A. et al. (2001), “Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila”, Proceedings of the Royal Society B: Biological Sciences, Vol. 268, No. 1485, pp. 2565-2570.
Mogi, M. and J. Mokry (1980), “Distribution of Wyeomyia smithii (Diptera: Culicidae) eggs in pitcher plants in Newfoundland, Canada”, Tropical Medicine, Vol. 22, pp. 1-12.
Muir, L.E., B.H. Kay and M.J. Thorne (1992), “Aedes aegypti (Diptera: Culicidae) vision: Response to stimuli from the optical environment”, Journal of Medical Entomology, Vol. 29, pp. 445-450.
Murrell, E.G. and A.J. Steven (2008), “Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae)”, Journal of Medical Entomology, Vol. 45, No. 3, pp. 375-385.
Nazni, W.A. et al. (2009), “Cross-mating between Malaysian strains of Aedes aegypti and Aedes albopictus in the laboratory”, The Southeast Asian Journal of Tropical Medicine and Public Health, Vol. 40, No. 1, pp. 40-46.
NCDENR (n.d.), Mosquitoes... Some Facts: Information Pamphlet, NC Dep. of Environment and Natural Resources, Div. of Environmental Health, Public Health Pest Management Section, Burlington North Carolina, United States, www.alamance-nc.com/envhealth/wp-content/uploads/sites/9/2013/10/Mosquitoes_Facts.pdf.
Nelson, M.J. (1986), Aedes aegypti: Biology and Ecology, Pan American Health Organization, Washington, DC, PNSP/86-63, pp. 50.
Nirgianaki, A. et al. (2003), “Wolbachia infections of the whitefly Bemisia tabaci”, Current Microbiology, Vol. 47, No. 2, pp. 93-101.
Nyasembe, V.O. et al. (2015), “Linalool oxide: Generalist plant-based lure for mosquito disease vectors”, Parasites and Vectors, Vol. 8, No. 581, pp. 1-8.
O’Gower, A.K. (1955), “The rate of digestion of human blood by certain species of mosquitoes”, Australian Journal of Biological Sciences, Vol. 9, No.1, pp. 125-129.
O’Neill, S.L. et al. (1997), “In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line”, Insect Molecular Biology, Vol. 6, pp. 33-39.
O’Neill, S.L. et al. (1992), “16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects”, PNAS, Vol. 89, pp. 2699-2702.
Pilger, D. et al. (2011), “Is routine dengue vector surveillance in central Brazil able to accurately monitor the Aedes aegypti population? Results from a pupal productivity survey”, Tropical Medicine & International Health, Vol. 16, No. 9, pp. 1143-1150.
Ponce, G.G. (1999), “Efecto de concentraciones subletales de Bacillus thuringiensis israelensis H-14 Vectobac® AS en parámetros biológicos de Aedes aegypti” [Effect of sublethal concentrations of Bacillus thuringiensis israelensis H-14 Vectobac® AS on biological parameters of Aedes aegypti], Masters thesis, Universidad Autonoma de Nuevo León, Facultad de Ciencias Biologicas.
Ponlawat, A. and L. Harrington (2009), “Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti”, American Journal of Tropical Medicine and Hygiene, Vol. 80, No. 3, pp. 395-400.
Ponlawat, A. and L. Harrington (2007), “Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae)”, Journal of Medical Entomology, Vol. 44, No. 3, pp. 422-426.
Ponlawat, A. and L.C. Harrington (2005), “Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand”, Journal of Medical Entomology, Vol. 42, No. 5, pp. 844-849.
Ponnusamy, L. et al. (2011), “Bacteria stimulate hatching of yellow fever mosquito eggs”, PLoS ONE, Vol. 6, No. 9: e24409.
Ramasamy, R. et al. (2011), “Larval development of Aedes aegypti and Aedes albopictus in peri-urban brackish water and its implications for transmission of arboviral diseases”, PLoS Neglected Tropical Diseases, Vol. 5, No. 11: e1369.
Reiter, P. et al. (1995), “Dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs”, The American Journal of Tropical Medicine and Hygiene, Vol. 52, pp. 177-179.
Richardson, J.B. et al. (2015), “Evidence of limited polyandry in a natural population of Aedes aegypti”, The American Journal of Tropical Medicine and Hygiene, Vol. 93, No. 1, pp. 189–193.
Rousset, F. et al. (1992), “Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods”, Proceedings of the Royal Society B: Biological Sciences, Vol. 250, No. 1328, pp. 91-98.
Rubio, A., M.V. Cardo and D. Vezzani (2011), “Tire-breeding mosquitoes of public health importance along an urbanization gradient in Buenos Aires, Argentina”, Memórias do Instituto Oswaldo Cruz, Vol. 106, No. 6, pp. 678-684.
Rueda, L.M. et al. (1990), “Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera. Culicidae)”, Journal of Medical Entomology, Vol. 27, No. 5, pp. 892-898.
Sánchez-Hernández, C. (2011), “Competencia larvaria interespecífica de Aedes aegypti y Aedes albopictus en condiciones de insectario en Tapachula”, Tesis de Licenciatura, Universidad Autónoma de Chiapas, Tapachula.
Saridaki, A. and K. Bourtzis (2010), “Wolbachia: More than just a bug in insects genitals”, Current Opinion in Microbiology, Vol. 13, pp. 67-72.
Scott, T.W. and W. Takken (2012), “Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission”, Trends in Parasitology, Vol. 28, No. 3, pp. 114-121.
Scott, T.W. et al. (2000), “Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Population dynamics”, Journal of Medical Entomology, Vol. 37, No. 1, pp. 77-88.
Scott, T.W. et al. (1993), “Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village”, Journal of Medical Entomology, Vol. 30, No. 5, pp. 922-927.
Service, M. (2012), Medical Entomology for Students, 5th Ed., Cambridge University Press, New York, pp. 303.
Shlenova, M.F. (1938), “[The speed of blood digestion in female A. maculipennis messeae at stable effective temperatures - Vitesse de la digestion du sang par la femelle de l'Anopheles maculipennis messeae aux temperatures effectives constantes] (in Russian)”, Med. Parazit. (Moscow), Vol. 7, pp. 716-735.
Siegel, J.P. et al. (1992), “Statistical appraisal of the weight-wing length relationship of mosquitoes”, Journal of Medical Entomology, Vol. 29, No. 4, pp. 711-714.
Silver, J.B. (2007), Mosquito Ecology: Field Sampling Methods, Springer Science and Business Media.
Sirot, L.K. et al. (2008), “Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: Potential tools for control of female feeding and reproduction”, Insect Biochemistry and Molecular Biology, Vol. 38, No. 2, pp. 176-189.
Sivagnaname, N. and K. Gunasekaran (2012), “Need for an efficient adult trap for the surveillance of dengue vectors”, Indian Journal of Medical Research, No. 136, No. 5, pp. 739-749.
Sota, T. and M. Mogi (1992), “Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size”, Oecologia, Vol. 90, pp. 354-358.
Stouthamer, R., J.A. Breeuwer and G.D. Hurst (1999), “Wolbachia pipientis: Microbial manipulator of arthropod reproduction”, Annual Review of Microbiology, Vol. 53, pp. 71-102.
Takken, W. (1991), “The role of olfaction in host-seeking of mosquitoes: A review”, Insect Science Applied, Vol. 12, No. 1-2-3, pp. 287-295.
Takken, W. et al. (1998), “Effect of body size on host seeking and bloodmeal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): The disadvantage of being small”, Journal of Medical Entomology, Vol. 35, pp. 639-645.
Tamayo-Domínguez, R. (2011), “Comparación del ciclo gonotrófico de hembras de Aedes aegypti Linnaeus, 1762 y Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) en condiciones de insectario en el Municipio de Tapachula, Chiapas” [Comparison of the gonotrophic cycle of females of Aedes aegypti Linnaeus, 1762 and Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) under insectary conditions in the Municipality of Tapachula, Chiapas], Degree thesis, Universidad Autónoma de Chiapas, Tapachula.
Thavara, U. et al. (2001), “Larval occurrence, oviposition behavior and biting activity of potential mosquito vector of dengue in Samui Island, Thailand”, Journal of Vector Ecology, Vol. 26, No. 2, pp. 172-180.
Thirión, I.J. (2003), El Mosquito Aedes aegypti y el Dengue en México [The Aedes aegypti Mosquito and Dengue in Mexico], Bayer Environmental Science, Bayer de México, S.A. de C. V., pp. 151.
Thomas, S.M. et al. (2012), “Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae)”, Parasites and Vectors, Vol. 5, No. 100.
Tripet, F. et al. (2011), “Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors”, The American Journal of Tropical Medicine and Hygiene., Vol. 85, No. 2, pp. 265-270.
Trpis, M. et al. (1973), “Diel periodicity in the landing of Aedes aegypti on man”, Bulletin of the World Health Organization, Vol. 48, pp. 623-629.
Tyagi, B.K. et al. (2006), “Dengue in Kerala: A critical review”, ICMR Bulletin, Vol. 36, No. 4-5, pp. 13-22.
Ulloa, A. et al. (2010), “Cement tank: An artificial water container for Aedes aegypti”, Journal of the American Mosquito Control Association, Vol. 26, No. 3, pp. 307.
Ulloa, G.A. (1996), “Abundancia larvaria y fuentes alimenticias de Aedes aegypti (L) (Diptera: Culicidae) en algunos recipientes artificiales en el sur de Chiapas, México” [Larval abundance and food sources of Aedes aegypti (L) (Diptera: Culicidae) in some artificial containers in southern Chiapas, Mexico], Science Masters Thesis in Medical Entomology, Universidad Autónoma de Nuevo León, San Nicolás de los Garza.
Van Handel, E. et al. (1994), “Plant-sugar, glycogen, and lipid assay of Aedes aegypti collected in urban Puerto Rico and rural Florida”, Journal of the American Mosquito Control Association, Vol. 10, pp. 149-153.
Vezzani, D. and N. Schweigmann (2002), “Suitability of containers from different sources as breeding sites of Aedes aegypti (L.) in a cemetery of Buenos Aires city, Argentina”, Memórias do Instituto Oswaldo Cruz, Vol. 97, No. 6, pp. 789-792.
Werren, J.H., L. Baldo and M.E. Clark (2008), “Wolbachia: Master manipulators of invertebrate biology”, Nature Reviews Microbiology, Vol. 6, pp. 741-751.
West, A.S. and G.S. Eligh (1952), “The rate of digestion of blood in mosquitoes. Precipitin test studies”, Canadian Journal of Zoology, Vol. 30, pp. 267-272.
Wheeler, D. (1996), “The role of nourishment in oogenesis”, Annual Review of Entomology, Vol. 41, pp. 407-431.
Winch, P.J. et al. (1992), “Variation in Aedes aegypti larval indices over a one-year period in a neighborhood of Merida, Yucatan, Mexico”, Journal of the American Mosquito Control Association, Vol. 8, No. 2, pp. 193-195.
Wong, J. et al. (2011), “Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control”, PLoS Neglected Tropical Diseases, Vol. 5, No. 4: e1015.
Ye, Y.H. et al. (2013), “Wolbachia-associated bacterial protection in the mosquito Aedes aegypti”, PLoS Neglected Tropical Diseases, Vol. 7, No. 8: e2362.
Yen, J.H. and A.R. Barr (1973), “New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L”, Nature, Vol. 232, pp. 657-658.