Chapter 1. Common Bean (Phaseolus vulgaris)
This chapter deals with the composition of common bean (Phaseolus vulgaris L.). It contains elements that can be used in a comparative approach as part of a safety assessment of foods and feeds derived from new varieties. Background is given on bean production worldwide, common bean processing for industrial canning and other uses for human and animal consumption, followed by appropriate varietal comparators and characteristics screened by breeders. Nutrients in common bean seed, as well as main anti-nutrients, toxicants and other constituents, are then detailed. The final sections suggest key constituents for whole-grain analysis of new common bean varieties for food use and for feed use.
This chapter was prepared by the OECD Working Group for the Safety of Novel Foods and Feeds, with Brazil as the lead country. It was initially issued in December 2015. FAOSTAT data on production and trade, including Table 1.1, have been updated.
Background
General description of common bean (Phaseolus vulgaris L.)
Common bean (Phaseolus vulgaris L.) is a major grain legume which is consumed worldwide for its edible seeds and pods (Heuzé et al., 2013) (Figures 1.1 and 1.2). Wild common bean [Phaseolus vulgaris L., tribe Phaseoleae, family Leguminosae (Schrire, 2005)] is present throughout Central and South America (Gepts and Debouck, 1991; Freytag and Debouck, 2002). All cultivated varieties grown in the world today originate from two independent domestication events of wild populations at different pre-Columbian times (Kaplan and Lynch, 1999; Piperno, 2012) in western Mexico (Kwak et al., 2009) and in central Peru (Chacón-Sánchez et al., 2005). Human selection has generated dozens of landraces in each region (Singh et al., 1991). After 1492, the common bean was taken to South-Western Europe (Rodiño et al., 2006), the Mediterranean region (Angioi et al., 2010), (mostly eastern) Africa (Westphal, 1974), parts of Asia (Zhang et al., 2008) and back to the Americas (Albala, 2007; Gepts et Bliss, 1988).
Given this geographical and ecological expansion, the common bean is known by a variety of names under generic “bean” terms such as “frijol” in Spanish-speaking Latin America, “feijão” in Brazil, “judia” in Spain and “haricot” in French (Voysest and Dessert, 1991).
The common bean is an herbaceous vine. While it is an annual and monocarpic plant, some of its most primitive forms and wild relatives are pluri-annual and polycarpic vines in montane forests in Mexico and Central America (Freytag and Debouck, 2002). Cultivars vary widely, with bush determinate and vining indeterminate growth habits, and are selected for earliness. Further description of the common bean taxonomy, centres of origin and diversity, reproductive biology, genetics, hybridisation and introgression, general interactions with other organisms (ecology), common pests and pathogens, and biotechnological developments can be found in the Consensus Document on the Biology of Common Bean (Phaseolus vulgaris L.) (OECD, 2015).
The common bean is typically cultivated in a mono-crop system and mechanically harvested (Figure 1.3). Although leaves and rarely flowers are consumed by humans (Purseglove, 1968), its main products are seeds, which are harvested either before or after physiological maturity as green pods such as snap beans (also known as “green beans”) or dry beans respectively. Both forms have given rise to an important canning industry and, recently, frozen dried food products have also appeared on world markets. Most dry bean varieties are consumed after boiling; grains of some landraces, mostly central Andean, are consumed after toasting (Tohme et al., 1995). Dried stems and pods have been used as hay for animal feeding (Hendry, 1918; Westphal, 1974).
Production
The common bean is produced in subtropical and tropical regions, most often by smallholders, and constitutes a major staple crop in both developing and developed countries. Mainly used for human consumption, the common bean is the most important grain legume in the human diet at global level. According to the Consultative Group on International Agricultural Research, the common bean provides protein, complex carbohydrates and valuable micronutrients for more than 300 million people in the tropics. In many areas, beans are the second most important source of calories after maize (CGIAR, n.d.).
Quantification of the world production of the common bean is difficult, first because a substantial part of the crop is consumed on-farm, with limited sale on local markets, and has not been documented. The second reason lies in the fact that some dry beans subject to national and/or international trade are not discriminated at the species level. As a result, a category reported as “pulses” or “beans” may include several legume species other than the common bean (P. vulgaris L.) such as other Phaseolus sp. beans and even some Vigna sp. (Lackey, 1981; Voysest, 1983; FAOSTAT, 2019). Finally, the diverse products of the common bean, while all derived from the same species, may be counted under different categories. For example, snap beans (green beans) may be tallied separately from dry beans (Voysest and Dessert, 1991).
According to FAO estimates, the global bean production (covering not only the common bean) has risen from 16.6 million tonnes (Mt) in 1988-90 (3-year-average) up to the record of 29.3 Mt in 2015-17. This significant growth results from the increase of both cultivation areas and yields over the past 30 years, with the Americas and Asia as the most important producing regions (Table 1.1). According to other sources, South America alone is producing 30% of the global common bean (Heuzé et al., 2013).
The five top producer countries of dry beans during the 2013-17 period were, in annual average, India (5.8 Mt), Myanmar (4.9 Mt), Brazil (3.0 Mt), the United States (1.3 Mt) and Mexico (1.2 Mt), followed in ranking order by the People’s Republic of China and several African countries: the United Republic of Tanzania, Uganda, Kenya and Ethiopia (FAOSTAT, 2019).1
Common beans are mainly consumed in countries where they are produced. Countries with the highest rates of bean consumption per capita (mostly in Central and South Americas, the Caribbean, East Africa and some Asian economies) produce beans and also import them at varying levels, depending on the harvest, for meeting internal demand. Considering the global imports and exports of dry beans between 2012 and 2016, it seems that 12% to 18% of the world annual production (around 3.9 Mt on average) is traded internationally. China, Myanmar and the United States are the main exporters, with India and the European Union being the largest importers (FAOSTAT, 2019).
Processing
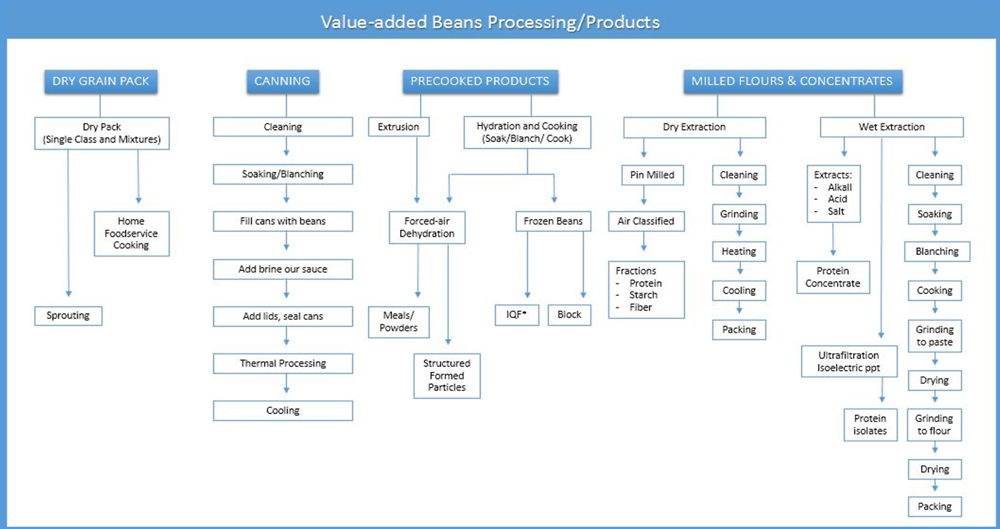
After harvest, beans are cleaned and then processed into final consumer products or ingredients. Products such as packaged dry beans, canned beans, baked beans, bean pastes, puffed snacks, texturised vegetable protein as meat analogues, cereal products, soups, frozen beans and bean flours all result from processing. The most commonly used processing methods for value-added common bean products are presented in Figure 1.4.
Canning is one of the most common forms of bean processing. Canned beans are a convenient alternative to dry beans which require long cooking times. An estimated 90% of navy beans and 45% of pinto beans (both types of common bean) consumed in the United States are sold as canned products (USDA-ERS, 2010). In developing countries, canned beans are most commonly a product for higher-income consumers (Jackson et al., 2012).
The canning process involves seven major steps (Figure 1.4):
-
First, seed sorting and cleaning are performed to remove poor quality, diseased and damaged seed, stones and debris.
-
Next, beans are equilibrated to 12%-16% moisture. Higher moisture values reduce the shelf life and lower values increase seed damage and splitting (Matella et al., 2012).
-
A soaking and/or blanching step follows. Soaking times may vary from 30 min to 12 h at room temperature. Blanching is high heat treatment for 30 min or less prior to canning. The purpose of both treatments is to increase the water content of the seeds and uniformity of the final product.
-
Beans are added to cans, followed by hot brine or sauce. Brine is a mixture of sugar, salt and calcium chloride. The calcium helps to maintain bean firmness. Sauces most commonly used in canning are tomato-based but there are many commercial products available with diverse flavour additives.
-
Lids are added to the cans, which are sealed and processed in a canning retort for 52 min to 325 min at 116 to 121°C, depending on can size and brine or sauce type.
-
Cans are cooled with water to an internal temperature of 38°C and are equilibrated for two weeks prior to use (Hosfield and Uebersax, 1980).
There are many ways to process the common bean into flour (Figure 1.4). One approach uses heat to inactivate the enzymes as a pre-cooking method. The steps include cleaning, soaking, blanching, cooking, grinding into a paste, drying, grinding again into flour, drying and packing. Another approach is dry milling, without pre-cooking the flour. In this case, bean seeds are ground into flour, followed by heating and packing. Both approaches generate breakfast and snack food products, as well as a texturing ingredient in tortilla chips, baked products, pasta and extruded products.
Uses
Although the major industrial food use of the common bean is canned beans, processing of the different types of bean through various treatments results in a range of ingredients for food and feed and value-added products: composite flour, extruded products, bread, cakes, pasta and tortillas and others, as presented in Table 1.2.
Appropriate comparators for testing new varieties
This document suggests parameters that common bean breeders should measure when developing new modified varieties of Phaseolus vulgaris. The data obtained in the analysis of a new common bean variety should ideally be compared to those obtained from an appropriate near-isogenic, non-modified variety, grown and harvested under the same conditions. The comparison can also be made between values obtained from new varieties and data available in the literature or chemical analytical data generated from other commercial common bean varieties.
Components to be analysed include key nutrients, anti-nutrients and toxicants. Key nutrients are those which have a substantial impact on the overall diet of humans (food) and animals (feed). These may be major constituents (fats, proteins, and structural and non-structural carbohydrates) or minor compounds (vitamins and minerals). Similarly, the levels of known anti-nutrients and allergens should be considered. Key toxicants are those toxicologically significant compounds known to be inherently present in the species, whose toxic potency and levels may impact human and animal health. Standardised analytical methods and appropriate types of material should be used, adequately adapted to the use of each product and by-product. The key components analysed are used as indicators of whether unintended effects of the genetic modification influencing plant metabolism have occurred or not.
Breeding characteristics screened by developers
The majority of common bean production occurs under low input agriculture on small-scale farms in developing countries (Miklas et al., 2006). Under such conditions, yield is mostly below its potential for the crop. Consequently, yield increase by attenuation of limiting factors is the focus of many breeding programmes (McClean et al., 2008).
Improving common bean nutritional quality, stress tolerance or resistance to pests and diseases are key objectives for various breeding programmes (Angenon et al., 1999; Suárez et al., 2008). Diseases and insects represent crucial biotic stressors that farmers have to face when growing this crop (Broughton et al., 2003). Among the fungal, bacterial and viral diseases that can affect common bean, at least five major ones are widespread: anthracnose, angular leaf spot, common bacterial blight, bean golden yellow mosaic virus and bean common mosaic virus, while several others are important locally or regionally (Broughton et al., 2003). A common bean variety that is resistant to bean golden mosaic virus (BGMV) has recently been developed (Aragão et al., 2013). Most commonly, breeders aim for resistance to one or two diseases and/or pest insects within the same variety. Since wild Phaseolus species present traits such as pest and pathogen resistance that are usually infrequent among cultivated common beans, they may be a potential source of novel alleles (Acosta-Gallegos et al., 2007).
The development of varieties with improved tolerance/resistant to other biotic stressors and to abiotic stressors is another important goal. Breeding programmes are developing agronomic traits such as nitrogen fixation. Other characteristics are also being explored by common bean breeding programmes, such as the increased content of specific nutrients including protein, minerals and vitamins.
Nutrients
Composition of common bean (Phaseolus vulgaris L.) – General points
This document addresses composition data relating to seeds only, not green pods (snap beans or green beans), dry shelled pods and stems.
The common bean is morphologically variable and adaptable to different environments, creating a wide range of local varieties. As a consequence, the nutritional composition of the common bean is impacted by various factors such as genotype, geographical origin, environmental and growing conditions (Broughton et al., 2003).
Constituents of common bean seed
Proximate composition
The proximate composition of raw common beans of a number of commercial varieties from Brazil, Madeira Island and the United States is shown in Table 1.3.
Carioca bean grains have a cream background with tan stripes; Pérola is the most common carioca variety in Brazil. The cooking process affects mainly the fibre content of Carioca beans (Pires et al., 2005).
Gouveia et al. (2014) evaluated the composition of 59 accessions of common bean varieties (52 Madeiran landraces, 5 standard and 2 commercial varieties) grown under the same field conditions in Madeira Island, to minimise the impact of the environmental factors. Regional common bean varieties exhibited great variability in the proximate parameters, presenting, on average, better nutritional performance with high protein and mineral contents compared to standard and commercial varieties.
Carbohydrates
Carbohydrates are monosaccharides and disaccharides (sugars), oligosaccharides and polysaccharides (starch, resistant starch and non-starch). Carbohydrates content in beans is mainly composed of starch, with small amounts of monosaccharides and disaccharides. Of carbohydrates, 17% to 23% has been reported to be pectin, cellulose and hemicellulose (Shiga et al., 2009). The total starch content ranges from 23.4% to 64.3% (Jacinto-Hernández and Campos, 1993; Jacinto-Hernández et al., 2002; Gouveia et al., 2014).
Beans contain a high ratio of slowly-digestible to readily-digestible starch compared with other starchy foods. Most common beans contain 27% to 40% amylose, a linear polymer of α-1-4 glucose units (Hoover et al., 2010), whereas most other starchy vegetables contain 20% to 30% amylose. Beans also contain a substantial amount of resistant starch, considered as dietary fibre. Resistant starch resists digestion by amylase in the small intestine and progresses to the large intestine for bacterial fermentation in the gut producing the short-chain fatty acids, acetic, butyric and propionic acids (Chung et al., 2010). Dry beans contain a substantial amount of carbohydrates as raw fibre in the form of cellulose and hemicellulose (Geil and Anderson, 1994).
Protein
Mean protein content shown for some common bean types in Table 1.3 varies from 23.27% to 26.72% dry matter. Madeira Island types/varieties had a protein mean content of 23.27 g/100 g with a range of 18.55 to 29.69 g/100 g (Gouveia et al., 2014). Bhatty et al. (2001) and Siddiq et al. (2010) reported a range of 20.43 to 23.62 g/100 g. Northern Portuguese beans and improved Ethiopian beans have been reported to contain total protein content ranging from 17.96 to 27.45 g/100 g (Coelho et al., 2005), and 17.96 to 22.07 g/100 g (Shimelis and Rakshit, 2005) respectively. Rodiño et al. (2001; 2003) have shown mean protein content of Portuguese beans and Iberian Peninsula beans to be 30.7 g/100 g (Rodiño et al., 2001) and 31.4 g/100 g respectively. Oliveira (2005) demonstrated that black, white and pink varieties have a protein content of 25% or more.
Table 1.4 presents the content of amino acids in common bean, based on elements collated from the USDA-ARS database (detailed by bean types, 2015), and the Feedipedia database (Heuzé et al., 2013). The amino acid profile of common bean protein is characterised by its deficiency in sulphur amino acids (methionine and cystine) and tryptophan, with methionine considered as the limiting amino acid. The amino acid most prevalent in all beans is glutamic acid (Table 1.4).
The protein digestibility of raw beans varies from 25% to 60% and can be increased up to 93.2%, depending on the bean variety and cooking process (Batista et al., 2010; Kiers et al., 2000; Jacinto-Hernández and Campos, 1993; Jacinto-Hernández et al., 2002). Jacinto-Hernández and Campos (1993) showed that increases in protein digestibility after cooking was very variable, with some varieties showing 8%-12% higher digestibility compared to raw beans, while others only improved digestibility by 3%-4%.
The nutritional value of beans is increased by heat processing, especially under moist heat (Gallardo et al., 1974, cited by Poel et al., 1990). This is due to denaturation of anti-nutritional factors, such as trypsin inhibitors and phytic acid (Burns, 1987, cited by Poel et al., 1990), and improved accessibility of the bean proteins to enzymatic degradation (Romero and Ryan, 1978).
Lipids/fatty acids
Beans contain only a small amount of lipids, with the majority of fatty acids being unsaturated (Anderson et al., 1999). Total fat/lipids content in some varieties of common beans ranges from 0.57 to 1.78 g/100 g of dry matter (Table 1.3). The total saturated, monounsaturated and polyunsaturated fatty acid contents of some types of common bean are presented in Table 1.5.
Vitamins
Common beans in particular contain water-soluble B vitamins; these include thiamine (3.9 to 11.4 mg/kg dry matter), riboflavin (1.0 to 2.9 mg/kg), niacin (3.3 to 26.8 mg/kg), vitamin B6 (0.4 to 5.7 mg/kg) and pantothenic acid (2.7 to 10.1 mg/kg) (Table 1.6). Common beans are also a prominent source of dietary folate – vitamin B9 – (0.2 to 5.8 mg/kg) (Table 1.6) (Rychlik et al., 2007). Common beans contain only small amounts of vitamin C, and little to no fat-soluble vitamins (Geil and Anderson, 1994) because of the low level of lipids in beans.
The vitamin content measured in common beans varies widely depending on commercial market classes, origin, environment and analytical methodology used for analysis (Table 1.6). Variation is greatest in folate (vitamin B9) content (Rychlik et al., 2007).
Cooking, like other food treatments, introduces another source of direct and indirect variability. Commercial methods of preparation of canned beans can cause significant loss of water-soluble vitamins, whereas home-cooked common beans seem to have less effect on nutrient retention (Augustin et al., 1981).
Minerals
The bean ash is constituted by several minerals (Table 1.7). The mineral content depends on market class/variety and environmental conditions during cultivation. Regarding minerals occurring at higher quantities, ranges reported are 0.09-4.25 g/kg of the dry matter for calcium (Ca), 1.0-3.26 g/kg for magnesium (Mg), 2.30-8.42 g/kg for phosphorous (P) and 13.0-24.9 g/kg for potassium (K). A considerable variation in levels was also observed for minerals occurring at lower quantities in germplasm from different sources, as shown by Dwivedi et al. (2012).
Common beans accumulate different proportions of iron, zinc and manganese in the seed coat, embryo and cotyledons. The highest amount of these minerals is stored in the cotyledons of mature seeds (Cvitanich et al., 2011). Iron and other constituents of the grain (phytate, tannins and fibre) are distributed differently in the hull and in the cotyledon. Food processing, such as baking and brewing, not only affect the bioavailability of iron but also factors that act as agonists or antagonists of mineral absorption (Lombardi-Boccia et al., 1995). Stripping significantly decreased the dialysability of iron, while cooking had the same influence on a coloured variety, but not on a white variety. The tannin-protein interaction may be the main cause of the difference in iron dialysability (Lombardi-Boccia et al., 1995). The effect of reheating beans on their iron content has also been studied. In whole bean, without broth, no changes were detected during cooking. In the case of beans with broth, insoluble iron increased in grains. Both soluble and insoluble iron decreased in the broth (Amaya et al., 1991).
Anti-nutrients, toxicants and other constituents
Anti-nutrients and toxicants – General points
In spite of good nutritional quality, common beans contain some constituents having anti-nutritional effects. Thus, adverse effects may be induced by tannins, phytates, protease inhibitors and lectins. Kidney beans have also been reported to contain toxic cyanogenic compounds (Cho et al., 2013) but only at trace levels having no health implications for the consumer.
Main anti-nutrients
Tannins
Tannins are colourless polyphenolic constituents of legumes (Reed, 1995). Levels reported in common bean varieties range from 10.1 to 44.2 mg catechin-equivalents per gramme dry weight (De Mejía et al., 2003, Helbig et al., 2003; Cruz-Bravo et al., 2011). Beans differ in content of tannins, which affect quality as they are converted into pigments visible during dehydration and oxidation. Tannins also have the ability to interact with proteins, resulting in reduced protein and mineral digestibility (Junk-Knievel et al., 2008). Condensed tannins are present in the dietary fibre fraction and can be considered indigestible or poorly digestible (Bartolomé et al., 1995). Cooking does not destroy tannins but they are partially removed with the cooking broth (Bressani and Elias, 1980). According to Ziena et al. (1991), less than 10% of total tannins are broken down during cooking, while about 50% are washed away in the cooking liquid.
Phytate/phytic acid
Phytic acid (also known as inositol hexakisphosphate (IP6), inositol polyphosphate, or phytate when in salt form) chelates mineral nutrients including calcium, magnesium, potassium, iron and zinc, rendering them unavailable to non-ruminant animals (NRC, 1998; Liener, 1994). Phytates are concentrated mostly in the cotyledons and embryo axes (up to 3% of total seed weight) of common bean (Kasim and Edwards, 1998; Blair et al., 2012) (Table 1.8). The negative effect on the bioavailability of minerals is associated with inositol penta- (IP5) and hexa-phosphate (IP6). Phytates also interact with basic protein residues and can inhibit digestive enzymes such as pepsin, pancreatin and amylase (Agostini and Ida, 2006).
Phytate content in common beans varies due to genetic differences between varieties, and environmental factors such as growing conditions, agricultural practices and location. Commonly reported levels are in the range 2.6-25.1 mg/g dry weight (Stanley and Aguilera, 1985; Estévez et al., 1991; Burbano et al., 1999; Helbig et al., 2003; Díaz-Batalla et al., 2006; Oomah et al., 2008; Martin-Cabrejas et al., 2009; Martinez Meyer et al., 2013; Pedrosa et al., 2015; Carvalho et al., 2015). In beans, phytate phosphorus constitutes a major portion of the total phosphorus content and is found preferentially in the cotyledon (Deshpande et al., 1982), accounting for 57%-81% of total phosphorus in Navy, 68%-72% in Red Kidney, 55-80% in Great Northern and 70% in California small white beans (Reddy, 2001). Low phytate bean germplasm has recently been developed (Campion et al., 2009). The proportion of phytate being IP5 and IP6, which are the most commonly detected inositol phosphate isomers, vary widely in raw beans. IP6 is the most predominant isomer, constituting from 64% (in Red Kidney beans) to 98% (in Pinto beans) of the total phytate content (Chen, 2004).
Of the various processing methods, fermentation and germination seem to be effective in decreasing the phytate concentration, while soaking and cooking can remove from 50% to more than 80% of endogenous phytate in beans (Sathe and Salunke, 1984).
Trypsin inhibitors
Common beans contain trypsin inhibitors which inhibit the digestive action of the trypsin enzyme. Trypsin inhibitor activity (TI) in uncooked beans have been reported to be in the range 6.3-55.2 trypsin inhibited units (TIU)/mg (Dhurandhar and Chang, 1990; Estévez et al., 1991; Jacinto-Hernández and Campos, 1993; Sotelo et al., 1995; De Mejía et al., 2003, 2005; Morales-de León et al., 2007; Olmedilla-Alonso et al., 2013; Pedrosa et al., 2015). The level of TI in the common bean is not only dependent on bean genotype but also on the environmental conditions where it was cultivated (De Mejía et al., 2003; 2005). In cooked beans, trypsin inhibitor activity is much lower than in raw beans (Jacinto-Hernández and Campos, 1993; Jacinto-Hernández et al., 2002; Morales-de León et al., 2007).
Alpha-amylase inhibitors
Common beans are the legume with the highest amount of alpha-amylase inhibitors. Alpha-amylase inhibitors inhibit the digestive enzyme α-amylase resulting in reduced digestibility of certain carbohydrates. Various types of α-amylase inhibitors have been described in the common bean (Ishimoto et al., 1995), including three different glycoprotein isoforms. Screening of 150 Brazilian bean varieties classified by colour revealed average values between 0.19 and 0.29 α-amylase inhibitor units per mg protein and a range between 0.09 and 0.40 α-amylase inhibitor units per mg protein (Table 1.9), with no correlation between inhibitory activity and seed coat colour (Lajolo et al., 1991).
Lectins
Lectins are proteins that bind to carbohydrate-containing molecules and are found in a variety of foods, including legumes such as the common bean (Gupta, 1987). The biological activity of lectins has been reviewed (Grant, 1991). Lectin levels reported vary with the methodology used for analysis. Several investigators reported levels between non-detectable and approximately 10 haemagglutinating units (HU) per gramme bean assayed with a method measuring haemagglutinating activity (Sotelo et al., 1995; De Mejía et al., 2003; 2005). Burbano et al. (1999), Olmedilla-Alonso et al. (2013) and Pedrosa et al. (2015) reported 0.3-165 mg/g dry weight using an indirect ELISA assay for phytohaemagglutinin quantification. Lectins have been shown to have growth inhibitory properties and result in toxicity in animals. The haemagglutinating activity of lectins can be reduced by moist-heat treatment (Gupta, 1987), making proper cooking prior to consumption an important step in the safe consumption of common beans (Ogawa and Date, 2014). Several cases of human toxicity due to ingestion of raw or under-cooked beans have been reported (Cornell University, 2014).
Other constituents
Oligosaccharides
Common bean varieties vary considerably in terms of their oligosaccharide content (Table 1.10), including the raffinose family oligosaccharides (RFOs). Thus, raffinose levels range from about non-detectable to 14.1 mg/g dry weight, stachyose from 0.9 to 63.8 mg/g and verbascose from non-detectable to a few mg/g, depending on the variety considered (Geil and Anderson, 1994; Weder et al., 1997; Burbano et al., 1999; Queiroz Kda et al., 2002; Díaz-Batalla et al., 2006; Campos-Vega et al., 2009; Cruz-Bravo et al., 2011; Kleintop et al., 2013; Olmedilla-Alonso et al., 2013; Slupski and Gebczynski, 2014; Pedrosa et al., 2015). Díaz-Batalla et al. (2006) noted that one out of fourteen studied common bean varieties contained exceptionally high levels of verbascose (35.8 mg/g dry weight). RFOs are broken down by the enzyme α-galactosidase which is not present in the lower gastrointestinal tract. As a result, RFOs are fermented by anaerobic bacteria in the gut, resulting in flatulence (Soccol, 2012). Soaking of dry beans prior to cooking is a common practice and has been shown to reduce the content of RFOs in common bean. The amount of raffinose and stachyose removed through soaking in Mexican common bean varieties was found to range from 7% to 60%, depending on the variety considered (Table 1.10).
Other carbohydrates in common bean include pectic substances, arabinogalactans and xyloglucans (Reddy et al., 1984; Sathe and Salunkhe, 1984). Like RFOs, these polysaccharides are subject to anaerobic fermentation (Geil and Anderson, 1994).
Saponins
Saponins are secondary plant metabolites that exist in a wide variety of edible legumes (Shi et al., 2004; Guajardo-Flores et al., 2012; Calvert et al., 1981). In common bean, they are particularly found in the seed coat. The most abundant saponin in the extracts of black bean seed coats is soyasaponin Af (Chavez-Santoscoy et al., 2013).
Phenolics
The major phenolic compounds of beans are simple phenolic acids and flavonoids. Highest phenolic content is found in the dark, highly pigmented bean varieties, in particular in their seed coat or hulls (Oomah et al., 2005) that are rich in flavonols, flavonoids, anthocyanins and tannins. Seed coat polyphenols are partly responsible for the post-harvest seed darkening and hard-to-cook phenomenon in beans (Marles et al., 2008; Campos-Vega et al., 2012). A single gene seems to control post-harvest darkening. In Pinto beans, the slow-darkening trait is controlled by a recessive allele (Junk-Knievel et al., 2008). Total phenolic content (50-1104 mg/kg) and the spectrum of the various phenolic constituents vary widely among and within market classes of common bean, depending on genetic and environmental factors.
The most abundant simple phenolic compounds in common beans are ferulic acid, sinapic acid, vanillic acid, caffeic acid, p-coumaric acid and p-hydroxybenzoic acid, their reported amounts varying with the methodology used for analysis. Syringic acid, chlorogenic acid, gallic acid and vanillin have also been reported to be present (Espinosa-Alonso et al., 2006: Luthria and Pastor-Corrales, 2006; Xu and Chang, 2009).
Kaempferol, often occurring with O- and C-glycosidic linkages, is the most abundant flavonol in beans with red beans and pinto beans containing greater amounts (14-209 and 148 mg/kg respectively) than black or grey beans (20 mg/kg) (Díaz-Batalla et al., 2006). Quercetin, another flavonol is present in black (9.7-23.5 mg/kg), cream-red (6.7-9.4 mg/kg) and grey (7.9 mg/kg) beans (Diaz-Batella et al., 2006).
Anthocyanins occurring in beans are simple, non-acylated anthocyanidins, usually containing glucose as the only sugar; however, malvidin 3-galactoside has been detected in black beans (Xu and Chang, 2009). Six different anthocyanidins have been detected in the (coloured) common bean but their relative percentage may differ among ecotypes and commercial market classes of beans. Several investigators reported delphinidin (49%-81%) to predominate, with petunidin (4%-32%), cyanidin (1%-23%) and malvidin (4%-14%) occurring at intermediate level, and pelargonidin (0.4%-6.5%) and peonidin (0.5%-3.7%) less frequently (Choung, 2005; Espinosa-Alonso et al., 2006; Xu and Chang, 2009). However, López et al. (2013) reported cyanidin and pelargonidin as the major anthocyanidins in dark beans, where they were complemented with small amounts of acylated delphinidin and pelargonidin 3-glucosides.
Suggested constituents to be analysed related to food use
Key products consumed by humans
The common bean is a staple food typically consumed after having been soaked in water and cooked, or after being canned. While beans can be milled and used to produce processed products, the common bean is typically eaten as shelled beans (whole grain). For the purpose of compositional analysis, it is appropriate to analyse the whole grain once the shell has been removed.
Suggested analysis for food use of new varieties
In the context of the human diet, the common bean can provide nutrients such as proteins, carbohydrates, dietary fibre and folate. The common bean may also contain anti-nutrients such as phytic acid, trypsin inhibitor, α-amylase inhibitor and lectins. The suggested key nutritional and anti-nutritional parameters to be analysed are shown in Table 1.11.
Suggested constituents to be analysed related to feed use
Key products consumed by animals
Although less common than its use in human food, the common bean may be used in animal feed. While products from the common bean may be used as feed, this document only addresses seeds, not green pods (snap beans or green beans), dry shelled pods and stems.
The residue of packaging and processing of dried beans (including those from the genera Phaseolus) for human food may be added to animal diets. These may include broken, small and cull beans, which may comprise all or part of plant protein products for animal diets (AAFCO, 2015).
Suggested analysis for feed use of new varieties
The suggested key nutritional and anti-nutritional parameters to be analysed in the common bean for animal feed use are shown in Table 1.12.
References
AAFCO (2015), 2015 Official Publications, The Association of American Feed Control Officials, http://www.aafco.org/Publications (accessed on 15 June 2015).
Acosta-Gallegos, J.A., J.D. Kelly and P. Gepts (2007), “Pre breeding in common bean and use of genetic diversity from wild germplasm”, Crop Science, Vol. 47(S3), pp. 44-59, https://www.researchgate.net/publication/242108033_Prebreeding_in_Common_Bean_and_Use_of_Genetic_Diversity_from_Wild_Germplasm.
Agostini, J.S. and E.I. Ida (2006),“Efeito das condições de germinação de girassol na redução do teor de fitato e ativação de fitase e fosfatase ácida (Effect of sunflower germination conditions for reducing phytate content and enhancing phytase and acid phosphatase activities)”, Ciências Agrárias, Vol. 27, No. 1, pp. 61-70, in Portuguese (English abstract), http://www.uel.br/revistas/uel/index.php/semagrarias/article/view/2398/2053.
Albala, K. (2007), Beans – A History, Berg Publishers, Oxford, UK.
Amaya, H., E. Acevedo and R. Bressani (1991), “Efecto del recalientamiento sobre la disponibilidad de hierro y del valor nutritivo de la proteína del frijol negro (Phaseolus vulgaris) cocido”, Archivos Latino Americanos de Nutricion, Vol. 41, No. 2, Caracas, pp. 222-237.
Anderson, J.W., B.M Smith and C.S. Washnock (1999), “Cardiovascular and renal benefits of dry bean and soybean intake”, American Journal of Clinical Nutrition, Vol. 70(suppl), pp. 464-474.
Angenon, G. et al. (1999), “Strategies for improving the nutritional quality of Phaseolus beans through gene engineering”, Biotechnology, Agronomy, Society and Environment, Vol. 3(4), pp. 233-236, http://www.pressesagro.be/base/text/v3n4/233.pdf.
Angioi, S.A. et al. (2010), “Beans in Europe: Origin and structure of the European landraces of Phaseolus vulgaris L.”, Theoretical and Applied Genetics, Vol. 121(5), Springer, pp. 829-843.
Aragão, F.J.L. et al. (2013), “Molecular characterization of the first commercial transgenic common bean immune to the Bean golden mosaic virus”, Journal of Biotechnology, Vol. 166, pp. 42-50.
Augustin, J. et al. (1981), “Variation in the vitamin and mineral content of raw and cooked commercial Phaseolus vulgaris classes”, Journal of Food Science, Vol. 46, Wiley, pp. 1701-1706, http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1981.tb04467.x/pdf.
Bartolomé, B., L.M. Jiménez-Ramsey and L.G. Butler (1995), “Nature of the condensed tannins present in the dietary fibre fractions in foods”, Food Chemistry, Vol. 53, No. 4, Barking, pp. 357-362.
Batista, K.A., S.H. Prudêncio and K.F. Fernandes (2010), “Changes in the functional properties and anti-nutritional factors of the extruded hard-to-cook common beans (Phaseolus vulgaris, L.)”, Journal of Food Science Oxford, Vol. 75, No. 3, pp. 286-290.
Bhatty, N., A.H. Gilani and S.A. Nagra (2001), “Nutritional Improvement of Lobia (Phaseolus vulgaris) by supplementation with poultry, mutton and beef meat”, International Journal of Food Sciences and Nutrition, Vol. 52, pp. 521-526.
Blair, M.W. et al. (2012), “Inheritance of seed phytate and phosphorous levels in common bean (Phaseolus vulgaris L.) and association with newly-mapped candidate genes”, Molecular Breeding, Vol. 30, pp. 1265-1277.
Blair, M.W. et al. (2010), “Registration of high mineral common bean germplasm lines NUA35 and NUA56 from the red-mottled seed class”, Journal of Plant Registrations, Vol. 4(1), pp. 55-59.
Bressani, R. and L.G. Elias (1980), “The nutritional role of polyphenols in beans”, in J.H. Hulse (ed.), Polyphenols in Cereals and Legumes, IDRC, Ottowa, pp. 61-72.
Broughton, W.J. et al. (2003), “Beans (Phaseolus spp.) – Model food legumes”, Plant & Soil, Vol. 252(1), pp. 55-128, https://www.researchgate.net/publication/226474316_Beans_Phaseolus_spp_-_Model_food_legumes.
Burbano, C. et al. (1999), “Evaluation of antinutritional factors of selected varieties of Phaseolus vulgaris”, Journal of the Science of Food and Agriculture, Vol. 79, pp. 1468-1472, http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0010(199908)79:11%3C1468::AID-JSFA387%3E3.0.CO;2-G/pdf.
Calvert, G.D. et al. (1981), “A trial of the effects of soya-bean flour and soya-bean saponins on plasma lipids, faecal bile acids and neutral sterols in hypercholesterolaemic men”, British Journal of Nutrition, Vol. 45(2), pp. 277–281.
Campion, B. et al. (2009), “Isolation and characterization of an LPA (low phytic acid) mutant in common bean (Phaseolus vulgaris L.)”, Theoretical and Applied Genetics, Vol. 118, pp. 1211–1221, http://link.springer.com/article/10.1007%2Fs00122-009-0975-8.
Campos-Vega, R., H.A. Vergara-Castañeda and B.D. Oomah (2012), “Functional food sources: Beans in sight”, in E. Popescu and I. Golubev (eds.), Beans: Nutrition, Consumption and Health, Ch.1, pp. 1-56, Nova Science Publishers Inc., New York, NY.
Campos-Vega, R. et al. (2009), “Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.)”, Journal of Food Science, Vol. 74(7), pp. T59-T65.
Carvalho, J.L.V. et al. (2015), “Comparative analysis of nutritional compositions of transgenic RNAi-mediated virus-resistant bean (event EMB-PV051-1) with its non-transgenic counterpart”, Transgenic Research, Vol. 24(5), pp. 813-819, http://link.springer.com/article/10.1007%2Fs11248-015-9877-5#.
Carvalho, L.M.J. et al. (2012), “Iron and zinc retention in common beans (Phaseolus vulgaris L.) after home cooking”, Food and Nutrition Research, Vol. 56, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3292239/.
CGIAR (n.d.), Common Bean Factsheet, Consultative Group on International Agricultural Research, http://www.cgiar.org/our-strategy/crop-factsheets/beans/ (accessed on 15 June 2015).
Chacón-Sánchez, M.I., B. Pickersgill and D.G. Debouck (2005), “Domestication patterns in common bean (Phaseolus vulgaris L.) and the origin of the Mesoamerican and Andean cultivated races”, Theoretical Applied Genetics, Vol. 110(3), pp. 432-444, http://link.springer.com/article/10.1007%2Fs00122-004-1842-2.
Chavez-Santoscoy, R.A., J.A. Gutierrez-Uribe and S.O. Serna-Saldivar (2013), “Effect of flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats as cholesterol micelle disruptors”, Plant Foods for Human Nutrition, Vol. 68(4), pp. 416-423, http://link.springer.com/article/10.1007%2Fs11130-013-0384-7.
Chen, Q. (2004), “Determination of phytic acid and inositol pentakisphosphates in foods by high-performance ion chromatography”, Journal of Agricultural and Food Chemistry, Vol. 52(15), pp. 4604-4613.
Cho, H.-J. et al. (2013), “Determination of cyanogenic compounds in edible plants by ion chromatography”, Toxicological Research, Vol 29(2), pp. 143-147, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834451/.
Choung, M.G. (2005), “Structural analysis of black common bean (Phaseolus vulgaris L.) anthocyanins”, Food Science and Biotechnology, Vol. 14(5), pp. 672-675.
Chung, H.J., Q. Liu, and R. Hoover (2010), “Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches”, Food Research International, Vol. 43(2), pp. 501-508, http://www.sciencedirect.com/science/article/pii/S0963996909002154.
Codex Alimentarius Commission (2003), Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant DNA Plants – CAC/GL 45/2003, Annexes II and III adopted in 2008, www.codexalimentarius.net/download/standards/10021/CXG_045e.pdf.
Coelho, C.M.M., S.M. Tsai and V.A. Vitorello (2005), “Dynamics of inositol phosphate pools (tris-, tetrakis- and pentakisphosphate) in relation to the rate of phytate synthesis during seed development in common bean (Phaseolus vulgaris)”, Journal of Plant Physiology, Vol. 162, pp. 1-9, http://www.sciencedirect.com/science/article/pii/S0176161704001543.
Cornell University (2014), Plant Poisonous to Livestock – Plant Lectins, Department of Animal Science, Cornell University, http://www.ansci.cornell.edu/plants/toxicagents/lectins.html (accessed on 20 March 2015).
Cruz-Bravo, R.K. et al. (2011), “Fermented nondigestible fraction from common bean (Phaseolus vulgaris L.) cultivar Negro 8025 modulates HT-29 cell behavior”, Journal of Food Science, Vol. 76(2), pp. T41-T47, http://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2010.02025.x/pdf.
Cvitanich, C. et al. (2011), “Micro-PIXE investigation of bean seeds to assist micronutrient biofortification”, Nuclear Instruments and Methods in Physics Research Section B, Vol. 269(20), pp. 2297-2302, http://www.sciencedirect.com/science/article/pii/S0168583X11002308.
De Mejía, E. et al. (2005), “Tannins, trypsin inhibitors and lectin cytotoxicity in tepary (Phaseolus acutifolius) and common (Phaseolus vulgaris) beans”; Plant Foods Human Nutr. Vol. 60, pp. 137-145, http://link.springer.com/article/10.1007%2Fs11130-005-6842-0
De Mejía, E. et al. (2003), “Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semi-arid highlands of Mexico”, Journal of Agriculture and Food Chemistry, Vol. 51(20), pp. 5962–5966.
Delfino, R.A. and S.G. Canniatti-Brazaca (2010), “Interação de polifenóis e proteínas e o efeito na digestibilidade proteica de feijão comum (Phaseolus vulgaris L.) cultivar Pérola (Polyphenol and protein interaction and the effect on protein digestibility in common bean (P. vulgaris L.) cultivar Pérola)”, Ciência e Tecnologia de Alimentos, Vol. 30(2), Campinas, pp. 308-312, in Portuguese, http://www.scielo.br/pdf/cta/v30n2/03.pdf.
Deshpande, S.S. et al. (1982), “Effects of dehulling on phytic acid, polyphenols and enzyme inhibitors of dry beans (Phaseolus vulgaris L.)”, Journal of Food Science, Chicago, Vol. 47, No. 6, pp. 1846-1850, http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1982.tb12896.x/pdf.
Dhurandhar, N.V. and K.C. Chang (1990), “Effect of cooking on firmness, trypsin inhibitors, lectins and cystine/cysteine content of navy and red kidney beans (Phaseolus vulgaris)”, Journal of Food Science, Vol. 55(2), pp. 470–474, https://doi.org/10.1111/j.1365-2621.1990.tb06789.x.
Díaz-Batalla, L. et al. (2006), “Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.)”, Journal of Agricultural and Food Chemistry, Vol. 54(6), pp.2045-2052.
Dwivedi, S.L. et al. (2012), “Nutritionally enhanced stable food crops”, Plant Breeding Reviews, Vol. 36, pp 169-291.
Espinosa-Alonso, L.G. et al. (2006), “Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.)”, Journal of Agricultural and Food Chemistry, Vol. 54(12), pp. 4436-4444.
Estévez, A.M. et al. (1991), “Effect of processing on some chemical and nutritional characteristics of pre-cooked and dehydrated legumes”, Plant Foods for Human Nutrition, Vol. 41, pp. 193-201, http://link.springer.com/article/10.1007%2FBF02196387#.
FAOSTAT (2019), “Crops – Beans, dry – Production quantity, years 1988 to 2017 – Export and import quantities, years 2012 to 2016; Food supply quantity, bean, kg/capita, year 2013”, FAO Statistics Database, Food and Agriculture Organisation of the United Nations (FAO), http://faostat.fao.org (accessed on 10 July 2019).
Freytag, G.F. and D.G. Debouck (2002), “Taxonomy, distribution, and ecology of the genus Phaseolus (Leguminosae-Papilionoideae) in North America, Mexico and Central America”, Sida,Botanical Miscellany, Vol. 23, pp. 1-300.
Geil, P.B. and J.W. Anderson (1994), “Nutrition and health implications of dry beans: a review”, Journal of the American College of Nutrition, Vol. 13, No. 6, New York, pp. 549-558.
Gepts, P. and D.G. Debouck (1991), “Origin, domestication, and evolution of the common bean (Phaseolus vulgaris L.)”, in A. van Schoonhoven and O. Voysest (eds.), Common Beans: Research for Crop Improvement, Commonwealth Agricultural Bureaux International, Wallingford, UK, pp. 7-53.
Gepts, P. and F.A. Bliss (1988), “Dissemination pathways of common bean (Phaseolus vulgaris, Fabaceae) deduced from phaseolin electrophoretic variability. II: Europe and Africa”, The Americas Economic Botany, Vol. 42(1), pp. 73-85, http://link.springer.com/article/10.1007%2FBF02859038#page-2.
Gouveia, C.S.S. et al. (2014), “Nutritional and mineral variability in 52 accessions of common bean varieties (Phaseolus vulgaris L.) from Madeira Island”, Agricultural Sciences 2014, Vol. 5, pp. 317-329, https://doi.org/10.4236/as.2014.54034.
Grant, G. (1991), “Lectins”, in J.P.F. D’Mello, C.M. Duffus and J.H. Duffus (eds.), Toxic Substances in Crop Plants, The Royal Society of Chemistry, Cambridge, pp. 49-67.
Guajardo-Flores, D. et al. (2012), “Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans”, Food Chemistry, Vol. 134, pp. 1312-1319, http://www.sciencedirect.com/science/article/pii/S0308814613004561#.
Gupta, Y.P. (1987), “Anti-nutritional and toxic factors in food legumes: A review”, Plant Foods for Human Nutrition, Vol. 37(3), pp. 201-228, http://link.springer.com/article/10.1007%2FBF01091786#.
Guzmán-Maldonado, H.S. et al. (2002), “Calidad alimentaria y potencial nutraceutico del frijol (Phaseolus vulgaris L.) (Food quality and nutraceutical potential of common bean [Phaseolus vulgaris L.])”, Agricultura técnica en México, Vol. 28(2), pp. 159-173, in Spanish (English Abstract), http://www.redalyc.org/articulo.oa?id=60828206.
Helbig, E. et al. (2003), “Effect of soaking prior to cooking on the levels of phytate and tannin of the common bean (Phaseolus vulgaris, L.) and the protein value”, Journal of Nutritional Science and Vitaminology, Vol. 49, pp. 81-86.
Hendry, G.W. (1918), “Bean culture in California”, Bulletin of the University of California Agricultural Experimental Station, Vol. 294, pp. 285-347, http://babel.hathitrust.org/cgi/pt?id=uc2.ark:/13960/t2r50q843.
Heuzé, V. et al. (2013), Common Bean (Phaseolus vulgaris), Feedipedia.org – Animal Feed Resources Information System – A programme by INRA, CIRAD, AFZ and FAO, http://www.feedipedia.org/node/266 (accessed on 23 March 2015).
Hoover, R. et al. (2010), “Composition, molecular structure, properties, and modification of pulse starches: A review”, Food Research International, Vol. 43, pp. 399-413, http://www.sciencedirect.com/science/article/pii/S096399690900266X.
Hosfield, G.L. and M.A. Uebersax (1980), “Variability in physic-chemical properties and nutritional components of tropical and domestic dry bean germplasm”, Journal of the American Society for Horticultural Science, Vol. 105, pp. 246-252, http://onlinelibrary.wiley.com/doi/10.1002/jsfa.2740510302/pdf.
House, W.A. et al. (2002), “Potential for increasing the amounts of bioavailable zinc in dry beans (Phaseolus vulgaris L.) through plant breeding”, Journal of the Science of Food and Agriculture, Vol. 82(13), pp. 1452-1457, http://onlinelibrary.wiley.com/doi/10.1002/jsfa.1146/pdf.
Hu, Y. et al. (2006), “Kaempferol in red and pinto bean seed (Phaseolus vulgaris L.) coats inhibits iron bioavailability using an in vitro digestion/human Caco-2 cell model”, Journal of Agricultural and Food Chemistry, Vol. 54(24), pp. 9254-9261.
Ishimoto, M. et al. (1995), “Variation of seed α-amylase inhibitors in the common bean”, Theoretical and Applied Genetics, Vol. 90, pp. 425-429, http://link.springer.com/article/10.1007%2FBF00221985#.
Jacinto-Hernández, C. and E.A. Campos (1993), “Efecto de la cocción sobre algunas características nutricionales del frijol”, Revista Agronomía Mesoamericana, Vol. 4, pp. 42-47, in Spanish (English abstract), http://www.mag.go.cr/rev_meso/v04n01_042.pdf.
Jacinto-Hernández, C. et al. (2006), “Effect of soaking on the oligosaccharide content in common beans”, Annual Report of the Bean Improvement Cooperative BIC, Michigan State University, East Lansing, MI 48824, Vol. 49, pp. 159-160, http://naldc.nal.usda.gov/naldc/download.xhtml?id=IND43805445&content=PDF.
Jacinto-Hernández C. et al. (2002), “Caracterización de una población de líneas endogámicas de frijol común por su calidad de cocción y algunos componentes nutrimentales”, Agrociencia, Vol. 36, pp. 451-459, in Spanish, http://www.redalyc.org/articulo.oa?id=30236406.
Jackson, J. et al. (2012), “Utilization of dry beans and pulses in Africa”, in M. Siddiq and M.A. Uebersax (eds.), Dry Beans and Pulses Production, Processing and Nutrition, Blackwell Publishing Ltd., Oxford, UK, https://doi.org/10.1002/9781118448298.
Junk-Knievel, D.C., A. Vandenberg and K.E. Bett (2008), “Slow darkening in pinto bean (Phaseolus vulgaris L.) seed coats is controlled by a single major gene”, Crop Science, Vol. 48(1), pp. 189-193.
Kaplan, L. and T. Lynch (1999), “Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-Colombian Agriculture”, Economic Botany, Vol. 53(3), pp. 261-272, http://link.springer.com/article/10.1007%2FBF02866636#.
Kasim, A.B. and W.M. Edwards (1998), “The analysis for inositol phosphate forms in feed ingredients”, Journal of the Science of Food and Agriculture, Vol. 76, Chicago, pp.1-9, http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0010(199801)76:1%3C1::AID-JSFA922%3E3.0.CO;2-9/pdf.
Kiers, J.L., R.M.J. Nout and F.M. Rombouts (2000), “In vitro digestibility of processed and fermented soya bean, cowpea and maize”, Journal of the Science of Food and Agriculture, Vol. 80, No. 9, pp. 1325–1331, http://onlinelibrary.wiley.com/doi/10.1002/1097-0010(200007)80:9%3C1325::AID-JSFA648%3E3.0.CO;2-K/pdf.
Kleintop, A.E. et al. (2013), “Adaptation of the AOAC 2011.25 integrated total dietary fiber assay to determine the dietary fiber and oligosaccharide content of dry edible beans”, Journal of Agricultural and Food Chemistry, Vol. 61, pp. 9719-9726.
Kwak, M., J.A. Kami and P. Gepts (2009), “The putative Mesoamerican domestication center of Phaseolus vulgaris is located in the Lerma-Santiago basin of Mexico”, Crop Science, Vol. 49(2), pp. 554-563.
Lackey, J.A. (1981), “Tribe 10 – Phaseoleae DC”, in R.M. Polhill & P.H. Raven (eds.), Advances in Legume Systematics, Royal Botanic Garden, Kew, England, pp. 301-327.
Lajolo, F.M., F. Finardi-Filho and E.W. Menezes (1991), “Amylase inhibitors in Phaseolus vulgaris beans”, Food Technology, Vol. 45, pp. 119-121.
Liener, I.E. (1994), “Implications of antinutritional components in soybean foods”, Critical Reviews of Food Science and Nutrition, Vol. 34, No.1, pp. 31-67.
Lombardi-Boccia, G. et al. (1995), “Impact of processing on FE dialysability from bean (Phaseolus vulgaris L.)”, Food Chemistry, Vol. 53, No. 2, London, pp. 191-195.
López, A. et al. (2013), “Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.)”, Food Chemistry, Vol. 138(1), pp. 547-555, http://www.sciencedirect.com/science/article/pii/S0889157504001218.
Luthria, D.L. and M.A. Pastor-Corrales (2006), “Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties”, Journal of Food Composition and Analysis, Vol. 19, pp. 205-211, http://www.sciencedirect.com/science/article/pii/S0889157505000992.
Marles, M.A.S., A. Vandenberg and K.E. Bett (2008), “Polyphenol oxidase activity and differential accumulation of polyphenolics in seed coats of pinto bean (Phaseolus vulgaris L.) characterize postharvest color changes”, Journal of Agricultural and Food Chemistry, Vol. 56(16), pp. 7049-7056.
Martin-Cabrejas, M.A. et al. (2009), “The impact of dehydration process on antinutrients and protein digestibility of some legume flours”, Food Chemistry, Vol. 114, pp. 1063-1068.
Martinez Meyer, M.R. et al. (2013), “Content of zinc, iron and their absorption inhibitors in Nicaraguan common beans (Phaseolus vulgaris L.)”, Food Chemistry, Vol. 136(1), pp. 87-93, http://www.sciencedirect.com/science/article/pii/S0308814612012204.
Maskus, H (2010), “Pulse processing, functionality and application”, Literature review, University of Winnipeg, Manitoba, Pulse Canada, pp. 1-146.
Matella, N.J., D.K. Mishra and K.D. Dolan (2012), “Hydration, blanching, and thermal processing of dry beans”, in M. Siddiq and M.A. Uebersax (eds.), Dry Beans and Pulses Production and Consumption, Blackwell Publishing Ltd., Oxford, UK, https://doi.org/10.1002/9781118448298.
McClean, P.E. et al. (2008), “Phaseolus vulgaris: A diploid model for soybean”, in G. Stacey (ed.), Genomics of Soybean, Springer, New York, https://www.researchgate.net/publication/226039599_Phaseolus_vulgaris_A_Diploid_Model_for_Soybean.
Miklas, P.N. et al. (2006), “Common bean breeding for resistance against biotic and abiotic stresses from classical MAS breeding”, Euphytica, Vol. 147, pp. 106-131, http://link.springer.com/article/10.1007%2Fs10681-006-4600-5#page-1.
Morales-de León, J.C. et al. (2007), “Preparation and characterization of protein isolate from fresh and hardened beans (Phaseolus vulgaris)”, Journal of Food Science, Vol. 72, pp. C96-C102, http://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2006.00244.x/pdf.
NRC (1998), Nutrient Requirements of Swine, Tenth Revised Edition, National Research Council, National Academy Press, Washington, DC, www.nap.edu/catalog/6016/nutrient-requirements-of-swine-10th-revised-edition (accessed on 15 June 2015).
OECD (2015), “Consensus Document on the Biology of Common Bean (Phaseolus vulgaris L.)”, Series on Harmonisation of Regulatory Oversight in Biotechnology, No. 59, OECD, Paris, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2015)47&doclanguage=en.
Ogawa, H. and K. Date (2014), “The ‘white kidney bean incident’ in Japan”, in J. Hirabayashi (ed.), Lectins: Methods and Protocols, Methods of Molecular Biology, Vol. 1200, pp. 39-45.
Oliveira, J.E.D. de (2005), “Feijão na alimentação/nutrição do brasileiro: ontem e amanhã”, in Congresso Nacional De Pesquisa De Feijão 8. 2005, Goiânia. Anais, Santo Antônio de Goiás: Embrapa Arroz e Feijão, Vol. 2, Document no. 182, pp. 1245-1254.
Olmedilla-Alonso, B. et al. (2013), “Composition of two Spanish common dry beans (Phaseolus vulgaris), ‘Almonga’ and ‘Curruquilla’, and their postprandial effect in type 2 diabetics”, Journal of the Science of Food and Agriculture, Vol. 93, pp. 1076-1082, http://onlinelibrary.wiley.com/doi/10.1002/jsfa.5852/pdf.
Oomah, B.D., C. Blanchard and P. Balasubramanian (2008), “Phytic acid, phytase, minerals, and antioxidant activity in Canadian dry bean (Phaseolus vulgaris L.) cultivars”, Journal of Agricultural and Food Chemistry, Vol. 56(23), pp. 11312-11319.
Oomah, B.D., A. Cardador-Martinez and G. Loarca-Piña (2005), “Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.)”, Journal of the Science of Food and Agriculture, Vol. 85(6), pp. 935-942, http://onlinelibrary.wiley.com/doi/10.1002/jsfa.2019/pdf.
Pedrosa, M.M. et al. (2015), “Effects of industrial canning on the proximate composition, bioactive compounds contents and nutritional profile of two Spanish common dry beans (Phaseolus vulgaris L.)”, Food Chemistry, Vol. 166, pp. 68-75, http://www.sciencedirect.com/science/article/pii/S0308814614008887.
Piperno, D.L. (2012), “New archaeobotanical information on early cultivation and plant domestication involving microplant (phytolith and starch grain) remains”, in P. Gepts et al. (eds.), Biodiversity in Agriculture – Domestication, Evolution, and Sustainability, Cambridge University Press, UK, pp. 136-159.
Pires, C.V. et al. (2005), “Composição físico-química de diferentes cultivares de feijão (Phaseolus vulgaris L.)”, Alimentos e Nutrição, Vol. 16, No. 2, Araraquara, pp. 157-162.
Poel, T.F.B. et al. (1990), “Thermal Inactivation of lectins and trypsin inhibitor activity during steam processing of dry beans (Phaseolus vulgaris) and effects on protein quality”, Journal of the Science of Food and Agriculture, Vol. 53, No. 2, pp. 215-228, http://onlinelibrary.wiley.com/doi/10.1002/jsfa.2740530209/pdf.
Purseglove, J.W. (1968), Tropical Crops – Dicotyledons 1., John Wiley & Sons Inc., New York.
Queiroz Kda, S. et al. (2002), “Soaking the common bean in a domestic preparation reduced the contents of raffinose-type oligosaccharides but did not interfere with nutritive value”, Journal of Nutritional Science and Vitaminology, Vol. 48(4), pp. 283-289.
Reddy, N.R. (2001), “Occurrence, distribution, content, and dietary intake of phytate”, in N.R. Reddy and S.K. Sathe (eds.), Food Phytates, Ch.3, CRC Press, Boca Raton, FL, pp. 28.
Reddy, N.R. et al. (1984), “Chemical, nutritional and physiological aspects of dry bean carbohydrates – a review”, Food Chemistry, Vol. 13, No. 1, London, pp. 25-68.
Reed, J.D. (1995), “Nutritional toxicology of tannins and related polyphenols in forage legumes”, Journal of Animal Science, Vol. 73, pp. 1516-1528, https://www.ncbi.nlm.nih.gov/pubmed/7665384.
Rodiño, A.P. et al. (2006), “Novel genetic variation in common bean from the Iberian Peninsula”, Crop Science, Vol. 46(6), pp. 2540-2546.
Rodiño, A.P. et al. (2003), “A core collection of common bean from the Iberian Peninsula”, Euphytica, Vol. 131, pp. 165-175, https://doi.org/10.1023/A:1023973309788.
Rodiño, A.P. et al. (2001), “Diversity of common bean (Phaseolus vulgaris L.) germplasm from Portugal”, Genetic Resources and Crop Evolution, Vol. 48, pp. 409-417, https://doi.org/10.1023/A:1012248002436.
Romero, J. and D.S. Ryan (1978), “Susceptibility of the major storage protein of the bean, Phaseolus vulgaris L., to in vitro enzymic hydrolysis”, Journal of Agricultural and Food Chemistry, Vol. 26(4), pp. 784-788.
Rychlik, M. et al. (2007), “Folate contents of legumes determined by optimized enzyme treatment and stable isotope dilution assays”, Journal of Food Composition and Analysis, Vol. 20(5), pp. 411-419.
Sathe, S.K. and D.K. Salunkhe (1984), “Technology of removal of unwanted components of dry beans”, CRC Critical Reviews in Food Science and Nutrition, Vol. 21, No. 3, Cleveland, pp. 263-287.
Schrire, B.D. (2005), “Tribe Phaseoleae”, in G. Lewis et al. (eds.), Legumes of the World, Royal Botanic Gardens, Kew, England, pp. 392-431.
Shi, J. et al. (2004), “Saponins from edible legumes: Chemistry, processing, and health benefits”, Journal of Medicinal Food, Vol. 7(1), pp. 67-78.
Shiga, T.M., B.R. Cordenunsi and F.M. Lajolo (2009), “Effect of cooking on non-starch polysaccharides of hard-to-cook beans”, Carbohydrate Polymers, Vol. 76, pp. 100-109, http://www.sciencedirect.com/science/article/pii/S0144861708004669.
Shimelis, E.A. and S.K. Rakshit (2005), “Proximate composition and physico-chemical properties of improved dry bean (Phaseolus vulgaris L.) varieties grown in Ethiopia”, Food Science and Technology International, Vol. 38, pp. 331-338, https://www.researchgate.net/publication/222082332_Proximate_composition_and_physico-chemical_properties_of_improved_dry_bean_Phaseolus_vulgaris_L_varieties_grown_in_Ethiopia.
Siddiq, M. et al. (2010), “Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours”, Journal of Food Science and Technology, Vol. 43, pp. 232-237, http://www.sciencedirect.com/science/article/pii/S0023643809002047.
Singh, S.P., P. Gepts and D.G. Debouck (1991), “Races of common bean (Phaseolus vulgaris, Fabaceae)”, Economic Botany, Vol. 45(3), pp. 379-396, http://link.springer.com/article/10.1007%2FBF02887079#.
Slupski J. and P. Gẹbczyński (2014), “Changes due to cooking and sterilization in low molecular weight carbohydrates in immature seeds of five cultivars of common bean”, International Journal of Food Sciences and Nutrition, Vol. 65(4), http://www.tandfonline.com/doi/pdf/10.3109/09637486.2013.869794.
Soccol, C.R. (2012), “Chapter 6: Advances and applications of galactosidases in food industry”, in A.K. Haghi (ed.), Food Science: Research and Technology, p.58.
Sotelo, A., H. Sousa and M. Sánchez (1995), “Comparative study of the chemical composition of wild and cultivated beans (Phaseolus vulgaris)”, Plant Foods for Human Nutrition, Vol. 47, pp. 93-100, http://link.springer.com/article/10.1007%2FBF01089257#.
Stanley, D.W. and J.M. Aguilera (1985), “A review of textural defects in cooked reconstituted legumes – The influence of structure and composition”, Journal of Food Biochemistry, Vol. 9, No. 4, Westport, pp. 277-323, http://onlinelibrary.wiley.com/doi/10.1111/j.1745-4514.1985.tb00355.x/pdf.
Suárez, R. et al. (2008), “Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia”, Molecular plant-microbe interaction, Vol. 21, No. 7, pp. 958-966, http://apsjournals.apsnet.org/doi/pdf/10.1094/MPMI-21-7-0958.
Tiwari, B. and N. Singh (2012), Pulse Chemistry and Technology, The Royal Society of Chemistry Publishing, Cambridge, UK.
Tohme, J. et al. (1995), “Variability in Andean Nuña common bean (Phaseolus vulgaris, Fabaceae)”, Economic Botany, Vol. 49(1), pp. 78-95, http://www.jstor.org/stable/4255694?seq=1#page_scan_tab_contents.
USDA-ARS (2015), National Nutrient Database for Standard Reference, Release 27 (revised) – Version May 2015, United States Department of Agriculture, Agricultural Research Service, http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 15 June 2015).
USDA-ARS (2014), National Nutrient Database for Standard Reference, Release 27, United States Department of Agriculture, Agricultural Research Service, http://ndb.nal.usda.gov/ndb/search/list (accessed on 29 July 2014).
USDA-ESR (2010), Dry Edible Beans, Historic Data, United States Department of Agriculture, Economic Research Service webpage (accessed on 20 December 2013, no longer available).
Voysest, O. (1983), Variedades de Frijol en América Latin y su Origen, Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia.
Voysest, O. and M. Dessert (1991), “Bean cultivars: Classes and commercial seed types”, in A. van Schoonhoven and O. Voysest (eds.), Common Beans: Research for Crop Improvement, Commonwealth Agricultural Bureaux International, Wallingford, UK, pp. 119-162.
Wang, N. and J.K. Daun (2004), The Chemical Composition and Nutritive Value of Canadian Pulses, Canadian Grain Commission, Grain Research Laboratory, pp. 1-85.
Weder, J.K.P. et al. (1997), “Antinutritional factors in Anasazi and other pinto beans (Phaseolus vulgaris L.)”, Plant Foods for Human Nutrition, Vol. 51, pp. 85-98, http://link.springer.com/article/10.1023%2FA%3A1007924931856#.
Westphal, E. (1974), “Pulses in Ethiopia, their taxonomy and agricultural significance”, Centre for Agricultural Publishing and Documentation, Vol. 815, Wageningen, The Netherlands, pp. 159-176, https://edepot.wur.nl/197905.
Xu, B. and S.K.C. Chang (2009), “Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing”, Journal of Agricultural and Food Chemistry, Vol. 57(11), pp. 4754-4764.
Zhang, X., M.W. Blair and S. Wang (2008), “Genetic diversity of Chinese common bean (Phaseolus vulgaris L.) landraces assessed with simple sequence repeat markers”, Theor. Appl. Genet, Vol. 117(4), pp. 629-640, http://link.springer.com/article/10.1007%2Fs00122-008-0807-2.
Ziena, H.M., M. Youssef and A.R. El-Mahdy (1991), “Amino acid composition and some anti-nutritional factors of cooked faba beans (Medamnins): Effects of cooking temperature and time”, Journal of Food Science, Vol. 56, No. 5, Chicago, pp. 1347-1349, http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1991.tb04769.x/pdf.
Note
← 1. The FAO figures for dry beans are not limited to common bean only and aggregate data of other Phaseolus species, and for several countries other types of beans classified as Vigna species.